Nutr Res Pract.
2020 Apr;14(2):109-116. 10.4162/nrp.2020.14.2.109.
Effect of saccharin on inflammation in 3T3-L1 adipocytes and the related mechanism
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, 119, Dandae-ro, Dongnam-gu, Cheonan-si, Chungnam 31116, Korea. wkkim@dankook.ac.kr
- 2Department of Food Science and Nutrition, Natural Nutraceuticals Industrialization Research Center, DanKook University, Chungnam 31116, Korea.
- KMID: 2471878
- DOI: http://doi.org/10.4162/nrp.2020.14.2.109
Abstract
- BACKGROUND/OBJECTIVES
Excessive intake of simple sugars induces obesity and increases the risk of inflammation. Thus, interest in alternative sweeteners as a sugar substitute is increasing. The purpose of this study was to determine the effect of saccharin on inflammation in 3T3-L1 adipocytes.
MATERIALS/METHODS
3T3-L1 preadipocytes were differentiated into adipocytes. The adipocytes were treated with saccharin (0, 50, 100, and 200 µg/mL) for 24 h. Inflammation was induced by exposure of treated adipocytes to lipopolysaccharide (LPS) for 18 h and cell proliferation was measured. The concentration of nitric oxide (NO) was measured by using Griess reagent. Protein expressions of nuclear factor kappa B (NF-κB) and inhibitor κB (IκB) were determined by western blot analysis. The mRNA expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), interleukin 1β (IL-1β), interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-α (TNF-α) were determined by real-time PCR.
RESULTS
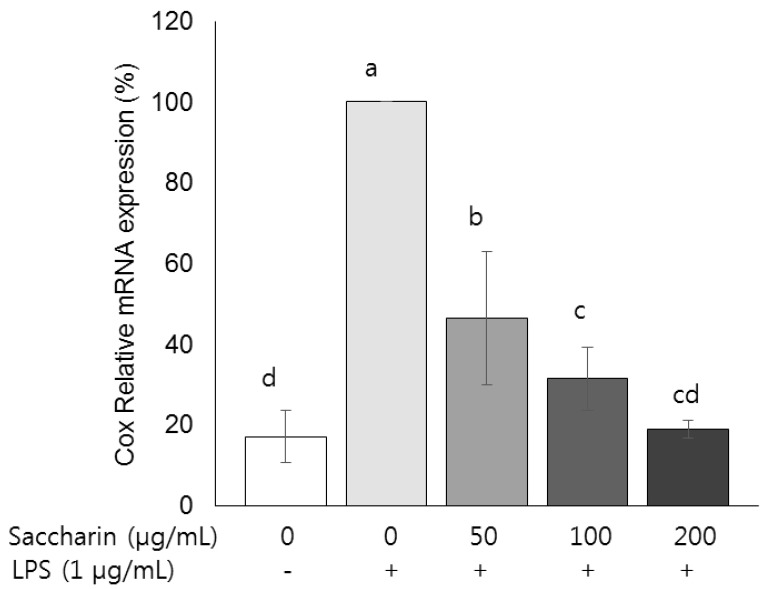
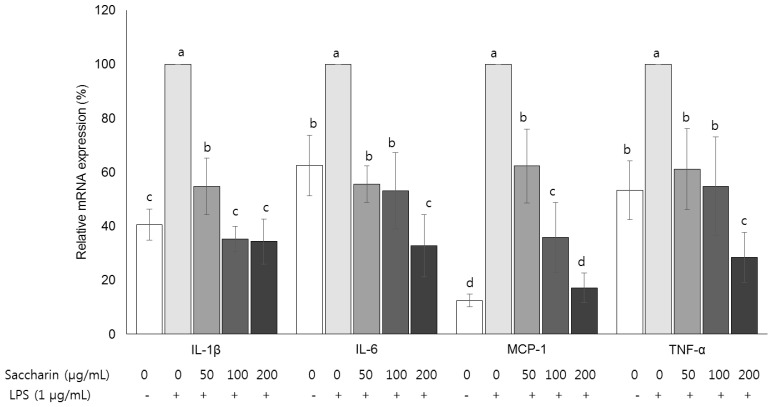
Compared with the control group, the amount of NO and the mRNA expression of iNOS in the LPS-treated group were increased by about 17.6% and 46.9%, respectively, (P < 0.05), and those parameter levels were significantly decreased by saccharin treatment (P < 0.05). Protein expression of NF-κB was decreased and that of IκB was increased by saccharin treatment (P < 0.05). Saccharin decreased the mRNA expression of COX-2 and the inflammation cytokines (IL-1β, IL-6, MCP-1, and TNF-α) (P < 0.05).
CONCLUSIONS
The results of this study suggest that saccharin can inhibit LPS-induced inflammatory responses in 3T3-L1 adipocytes via the NF-κB pathway.
Keyword
MeSH Terms
-
Adipocytes*
Blotting, Western
Carbohydrates
Cell Proliferation
Chemokine CCL2
Cyclooxygenase 2
Cytokines
Inflammation*
Interleukin-6
Interleukins
Necrosis
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Obesity
Real-Time Polymerase Chain Reaction
RNA, Messenger
Saccharin*
Sweetening Agents
Carbohydrates
Chemokine CCL2
Cyclooxygenase 2
Cytokines
Interleukin-6
Interleukins
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
RNA, Messenger
Saccharin
Sweetening Agents
Figure
Cited by 1 articles
-
Effects of quercetin on cell differentiation and adipogenesis in 3T3-L1 adipocytes
Seo Young Hong, Ae Wha Ha, Wookyoung Kim
Nutr Res Pract. 2021;15(4):444-455. doi: 10.4162/nrp.2021.15.4.444.
Reference
-
1. Aller EE, Abete I, Astrup A, Martinez JA, van Baak MA. Starches, sugars and obesity. Nutrients. 2011; 3:341–369. PMID: 22254101.
Article2. Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001; 357:505–508. PMID: 11229668.
Article3. Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010; 100:47–54. PMID: 20138901.
Article4. Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res. 2016; 306:1–7. PMID: 26970578.
Article5. Schäffler A, Müller-Ladner U, Schölmerich J, Büchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev. 2006; 27:449–467. PMID: 16684901.6. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005; 115:911–919. PMID: 15867843.
Article7. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009; 1:a000034. PMID: 20066092.8. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners - a review. J Food Sci Technol. 2014; 51:611–621. PMID: 24741154.
Article9. Ju DL. The efficacy and safety of non-nutritive sweeteners. J Korean Diabetes. 2015; 16:281–286.
Article10. Mondal S, Brankow DW, Heidelberger C. Enhancement of oncogenesis in C3H/10T1/2 mouse embryo cell cultures by saccharin. Science. 1978; 201:1141–1142. PMID: 684434.
Article11. Chowaniec J, Hicks RM. Response of the rat to saccharin with particular reference to the urinary bladder. Br J Cancer. 1979; 39:355–375. PMID: 36123.
Article12. Mortensen A. Sweeteners permitted in the European Union: safety aspects. Scand J Food Nutr. 2006; 50:104–116.
Article13. Kim JW, Baek HH. Safety of saccharin and its current status of regulation in the World. Korean J Food Sci Technol. 2011; 43:659–674.
Article14. Cohen SM, Arnold LL, Emerson JL. Safety of saccharin. Agro Food Ind Hi Tech. 2008; 19:24–28.15. Choi JS, Park SY, Yang MG, Lee DB, Lee TB, Heo JH, Lee MW, Kim SW. Estimation of anti-proliferative activity of saccharin against various cancer cell lines and MSCs. Korean J Clin Lab Sci. 2016; 48:169–175.
Article16. Park JM, Song MK, Kim YJ, Kim YJ. Effect of saccharin intake in restraint-induced stress response reduction in rats. J Korean Biol Nurs Sci. 2016; 18:36–42.
Article17. Lee LS. Saccharin and cyclamate inhibit binding of epidermal growth factor. Proc Natl Acad Sci U S A. 1981; 78:1042–1046. PMID: 6262753.
Article18. Thompson MM, Mayer J. Hypoglycemic effects of saccharin in experimental animals. Am J Clin Nutr. 1959; 7:80–85. PMID: 13626891.
Article19. Bailey CJ, Day C, Knapper JM, Turner SL, Flatt PR. Antihyperglycaemic effect of saccharin in diabetic ob/ob mice. Br J Pharmacol. 1997; 120:74–78. PMID: 9117102.
Article20. Csakai A, Smith C, Davis E, Martinko A, Coulup S, Yin H. Saccharin derivatives as inhibitors of interferon-mediated inflammation. J Med Chem. 2014; 57:5348–5355. PMID: 24897296.
Article21. Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, Sánchez-Lozada LG, Johnson RJ, Nakagawa T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008; 19:1712–1720. PMID: 18508964.
Article22. DiNicolantonio JJ, Mehta V, Onkaramurthy N, O'Keefe JH. Fructose-induced inflammation and increased cortisol: a new mechanism for how sugar induces visceral adiposity. Prog Cardiovasc Dis. 2018; 61:3–9. PMID: 29225114.
Article23. Cullberg KB, Larsen JO, Pedersen SB, Richelsen B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr Diabetes. 2014; 4:e113. PMID: 24662749.
Article24. Dobashi K, Asayama K, Nakane T, Kodera K, Hayashibe H, Nakazawa S. Troglitazone inhibits the expression of inducible nitric oxide synthase in adipocytes in vitro and in vivo study in 3T3-L1 cells and Otsuka Long-Evans Tokushima Fatty rats. Life Sci. 2000; 67:2093–2101. PMID: 11057759.25. Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010; 86:211–218. PMID: 20202975.26. Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med. 2013; 65:162–174. PMID: 23792277.
Article27. Boonkaewwan C, Burodom A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells. J Sci Food Agric. 2013; 93:3820–3825. PMID: 23794454.
Article28. Legler DF, Bruckner M, Uetz-von Allmen E, Krause P. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int J Biochem Cell Biol. 2010; 42:198–201. PMID: 19788928.
Article29. Lee W, Kang N, Park SY, Cheong SH, Chang KJ, Kim SH, Um JH, Han EJ, Kim EA, Jeon YJ, Ahn G. Xylose-taurine reduced suppresses the inflammatory responses in lipopolysaccharide-stimulated Raw264.7 macrophages. Adv Exp Med Biol. 2017; 975:633–642. PMID: 28849487.
Article30. Ballak DB, Stienstra R, Tack CJ, Dinarello CA, van Diepen JA. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine. 2015; 75:280–290. PMID: 26194067.
Article31. Su C, Chen M, Huang H, Lin J. Testosterone enhances lipopolysaccharide-induced interleukin-6 and macrophage chemotactic protein-1 expression by activating the extracellular signal-regulated kinase 1/2/nuclear factor-κB signalling pathways in 3T3-L1 adipocytes. Mol Med Rep. 2015; 12:696–704. PMID: 25738264.
Article32. Blancas-Flores G, Alarcón-Aguilar FJ, García-Macedo R, Almanza-Pérez JC, Flores-Sáenz JL, Román-Ramos R, Ventura-Gallegos JL, Kumate J, Zentella-Dehesa A, Cruz M. Glycine suppresses TNF-α-induced activation of NF-κB in differentiated 3T3-L1 adipocytes. Eur J Pharmacol. 2012; 689:270–277. PMID: 22732655.33. Kim SY, Jo MJ, Hwangbo M, Back YD, Jeong TY, Cho IJ, Jee SY. Anti-inflammatory effect of Stevia rebaudiana as a result of NF-κB and MAPK inhibition. J Korean Med Ophthalmol Otolaryngol Dermatol. 2013; 26:54–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Zaluzanin C Inhibits Differentiation of 3T3-L1 Preadipocytes into Mature Adipocytes
- Carnosic acid inhibits TLR4-MyD88 signaling pathway in LPS-stimulated 3T3-L1 adipocytes