Korean Circ J.
2020 May;50(5):395-405. 10.4070/kcj.2019.0416.
Mitochondrial Quality Control in the Heart: New Drug Targets for Cardiovascular Disease
- Affiliations
-
- 1Department of Biomedical Science and Engineering, Gwangju Institute of Science and Technology, Gwangju, Korea.
- 2Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon, Korea.
- 3Biomedical Institute for Convergence at SKKU (BICS), Sungkyunkwan University (SKKU), Suwon, Korea.
- 4Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- 5Division of Cardiovascular medicine, Department of Internal medicine, Dankook University College of Medicine, Dankook University Hospital, Cheonan, Korea.
- 6Division of Cardiology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. JANGYS1212@yuhs.ac
- KMID: 2471778
- DOI: http://doi.org/10.4070/kcj.2019.0416
Abstract
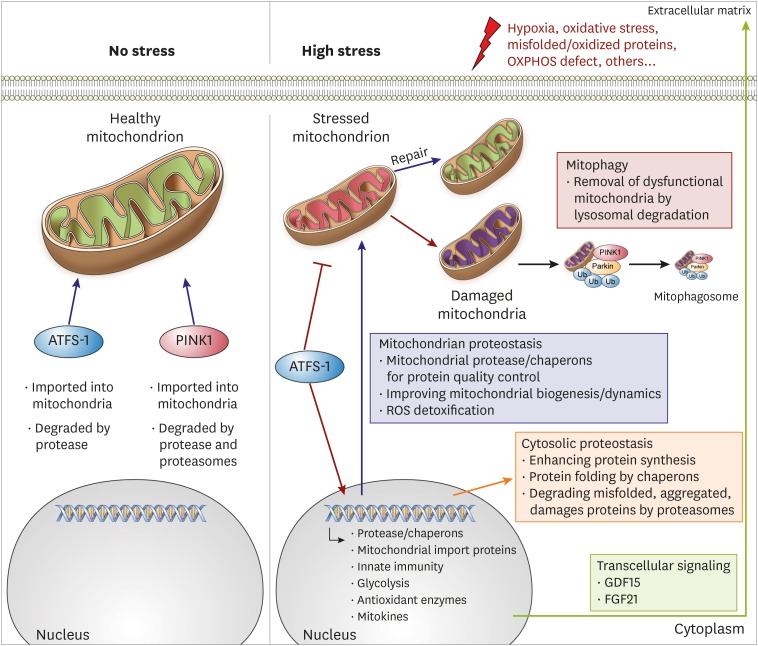
- Despite considerable efforts to prevent and treat cardiovascular disease (CVD), it has become the leading cause of death worldwide. Cardiac mitochondria are crucial cell organelles responsible for creating energy-rich ATP and mitochondrial dysfunction is the root cause for developing heart failure. Therefore, maintenance of mitochondrial quality control (MQC) is an essential process for cardiovascular homeostasis and cardiac health. In this review, we describe the major mechanisms of MQC system, such as mitochondrial unfolded protein response and mitophagy. Moreover, we describe the results of MQC failure in cardiac mitochondria. Furthermore, we discuss the prospects of 2 drug candidates, urolithin A and spermidine, for restoring mitochondrial homeostasis to treat CVD.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018; 128:3716–3726. PMID: 30124471.2. Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018; 28:R170–85. PMID: 29462587.3. Smyrnias I, Gray SP, Okonko DO, et al. Cardioprotective effect of the mitochondrial unfolded protein response during chronic pressure overload. J Am Coll Cardiol. 2019; 73:1795–1806. PMID: 30975297.4. Campos JC, Bozi LH, Bechara LR, Lima VM, Ferreira JC. Mitochondrial quality control in cardiac diseases. Front Physiol. 2016; 7:479. PMID: 27818636.5. Guaragnella N, Coyne LP, Chen XJ, Giannattasio S. Mitochondria-cytosol-nucleus crosstalk: learning from Saccharomyces cerevisiae. FEMS Yeast Res. 2018; 18:foy088.6. Goldman SJ, Taylor R, Zhang Y, Jin S. Autophagy and the degradation of mitochondria. Mitochondrion. 2010; 10:309–315. PMID: 20083234.7. Brown DA, Perry JB, Allen ME, et al. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017; 14:238–250. PMID: 28004807.8. Kuzmicic J, Del Campo A, López-Crisosto C, et al. Mitochondrial dynamics: a potential new therapeutic target for heart failure. Rev Esp Cardiol. 2011; 64:916–923. PMID: 21820793.9. Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol. 2013; 61:599–610. PMID: 23219298.10. Szklarczyk R, Nooteboom M, Osiewacz HD. Control of mitochondrial integrity in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130439. PMID: 24864310.11. Ren M, Phoon CK, Schlame M. Metabolism and function of mitochondrial cardiolipin. Prog Lipid Res. 2014; 55:1–16. PMID: 24769127.12. Moehle EA, Shen K, Dillin A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J Biol Chem. 2019; 294:5396–5407. PMID: 29622680.13. Yi HS. Implications of mitochondrial unfolded protein response and mitokines: a perspective on fatty liver diseases. Endocrinol Metab (Seoul). 2019; 34:39–46. PMID: 30912337.14. Ostermann J, Horwich AL, Neupert W, Hartl FU. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989; 341:125–130. PMID: 2528694.15. Höhfeld J, Hartl FU. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol. 1994; 126:305–315. PMID: 7913473.16. Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000; 275:3305–3312. PMID: 10652318.17. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018; 217:1915–1928. PMID: 29669742.18. Baker MJ, Tatsuta T, Langer T. Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol. 2011; 3:a007559. PMID: 21628427.19. Tondera D, Grandemange S, Jourdain A, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009; 28:1589–1600. PMID: 19360003.20. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012; 337:1062–1065. PMID: 22936770.21. Chinnery PF, Hudson G. Mitochondrial genetics. Br Med Bull. 2013; 106:135–159. PMID: 23704099.22. Melber A, Haynes CM. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018; 28:281–295. PMID: 29424373.23. Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013; 1:19–23. PMID: 23946931.24. Jang JY, Blum A, Liu J, Finkel T. The role of mitochondria in aging. J Clin Invest. 2018; 128:3662–3670. PMID: 30059016.25. Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012; 337:587–590. PMID: 22700657.26. Seiferling D, Szczepanowska K, Becker C, et al. Loss of CLPP alleviates mitochondrial cardiomyopathy without affecting the mammalian UPRmt . EMBO Rep. 2016; 17:953–964. PMID: 27154400.27. Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 2016; 26:2037–2043. PMID: 27426517.28. Wang YT, Lim Y, McCall MN, et al. Cardioprotection by the mitochondrial unfolded protein response requires ATF5. Am J Physiol Heart Circ Physiol. 2019; 317:H472–H478. PMID: 31274354.29. Quirós PM, Prado MA, Zamboni N, et al. Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J Cell Biol. 2017; 216:2027–2045. PMID: 28566324.30. Shires SE, Gustafsson ÅB. Mitophagy and heart failure. J Mol Med (Berl). 2015; 93:253–262. PMID: 25609139.31. Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010; 191:933–942. PMID: 21115803.32. Chan NC, Salazar AM, Pham AH, et al. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011; 20:1726–1737. PMID: 21296869.33. Nah J, Miyamoto S, Sadoshima J. Mitophagy as a protective mechanism against myocardial stress. Compr Physiol. 2017; 7:1407–1424. PMID: 28915329.34. Zhang H, Bosch-Marce M, Shimoda LA, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008; 283:10892–10903. PMID: 18281291.35. Zhang W, Ren H, Xu C, et al. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. eLife. 2016; 5:e21407. PMID: 27995894.36. Murakawa T, Yamaguchi O, Hashimoto A, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015; 6:7527. PMID: 26146385.37. Jovaisaite V, Mouchiroud L, Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 2014; 217:137–143. PMID: 24353213.38. Xu M, Xue RQ, Lu Y, et al. Choline ameliorates cardiac hypertrophy by regulating metabolic remodelling and UPRmt through SIRT3-AMPK pathway. Cardiovasc Res. 2019; 115:530–545. PMID: 30165480.39. Yarbrough WM, Mukherjee R, Ikonomidis JS, Zile MR, Spinale FG. Myocardial remodeling with aortic stenosis and after aortic valve replacement: mechanisms and future prognostic implications. J Thorac Cardiovasc Surg. 2012; 143:656–664. PMID: 21762938.40. Wang YT, Lim Y, McCall MN, Haynes CM, Nehrke KW, Brookes PS. Cardioprotection by the mitochondrial unfolded protein response (UPRmt) is mediated by activating transcription factor 5 (ATF5). bioRxiv. 2018; 344606.41. Nadtochiy SM, Wang YT, Nehrke K, Munger J, Brookes PS. Cardioprotection by nicotinamide mononucleotide (NMN): involvement of glycolysis and acidic pH. J Mol Cell Cardiol. 2018; 121:155–162. PMID: 29958828.42. Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017; 120:1812–1824. PMID: 28546358.43. Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A. 2011; 108:9572–9577. PMID: 21606348.44. Siddall HK, Yellon DM, Ong SB, et al. Loss of PINK1 increases the heart's vulnerability to ischemia-reperfusion injury. PLoS One. 2013; 8:e62400. PMID: 23638067.45. Kubli DA, Zhang X, Lee Y, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013; 288:915–926. PMID: 23152496.46. Shirakabe A, Zhai P, Ikeda Y, et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation. 2016; 133:1249–1263. PMID: 26915633.47. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018; 122:624–638. PMID: 29449364.48. Liang Q, Kobayashi S. Mitochondrial quality control in the diabetic heart. J Mol Cell Cardiol. 2016; 95:57–69. PMID: 26739215.49. Galloway CA, Yoon Y. Mitochondrial dynamics in diabetic cardiomyopathy. Antioxid Redox Signal. 2015; 22:1545–1562. PMID: 25738230.50. Tong M, Saito T, Zhai P, et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ Res. 2019; 124:1360–1371. PMID: 30786833.51. Wang S, Zhao Z, Feng X, et al. Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J Cell Mol Med. 2018; 22:5132–5144. PMID: 30063115.52. Hoshino A, Mita Y, Okawa Y, et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013; 4:2308. PMID: 23917356.53. Kubli DA, Quinsay MN, Gustafsson ÅB. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun Integr Biol. 2013; 6:e24511. PMID: 23986804.54. Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A. 2013; 110:8638–8643. PMID: 23650379.55. Diwan A, Krenz M, Syed FM, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007; 117:2825–2833. PMID: 17909626.56. Yussman MG, Toyokawa T, Odley A, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002; 8:725–730. PMID: 12053174.57. Cicero AF, Colletti A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine. 2016; 23:1134–1144. PMID: 26778479.58. Vasanthi HR, ShriShriMal N, Das DK. Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem. 2012; 19:2242–2251. PMID: 22414106.59. Vicinanza R, Zhang Y, Henning SM, Heber D. Pomegranate juice metabolites, ellagic acid and urolithin a, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid Based Complement Alternat Med. 2013; 2013:247504. PMID: 23710216.60. Ryu D, Mouchiroud L, Andreux PA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016; 22:879–888. PMID: 27400265.61. Sumner MD, Elliott-Eller M, Weidner G, et al. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005; 96:810–814. PMID: 16169367.62. Aviram M, Rosenblat M. Pomegranate protection against cardiovascular diseases. Evid Based Complement Alternat Med. 2012; 2012:382763. PMID: 23243442.63. Savi M, Bocchi L, Mena P, et al. In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2017; 16:80. PMID: 28683791.64. Wu X, Zhu X, Zhou Y. Urolithin a suppress cardiac fibrosis via autophagy pathway in the diabetic cardiomyopathy. Circ Res. 2019; 125:A531.65. Andreux PA, Blanco-Bose W, Ryu D, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019; 1:595–603.66. Larqué E, Sabater-Molina M, Zamora S. Biological significance of dietary polyamines. Nutrition. 2007; 23:87–95. PMID: 17113752.67. Lenzen S, Hickethier R, Panten U. Interactions between spermine and Mg2+ on mitochondrial Ca2+ transport. J Biol Chem. 1986; 261:16478–16483. PMID: 3782131.68. Jing YH, Yan JL, Wang QJ, et al. Spermidine ameliorates the neuronal aging by improving the mitochondrial function in vitro. Exp Gerontol. 2018; 108:77–86. PMID: 29649571.69. Fan J, Yang X, Li J, et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget. 2017; 8:17475–17490. PMID: 28407698.70. Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016; 22:1428–1438. PMID: 27841876.71. Tong D, Hill JA. Spermidine promotes cardioprotective autophagy. Circ Res. 2017; 120:1229–1231. PMID: 28408448.72. Madeo F, Bauer MA, Carmona-Gutierrez D, Kroemer G. Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy. 2019; 15:165–168. PMID: 30306826.73. Stegemann C, Pechlaner R, Willeit P, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014; 129:1821–1831. PMID: 24622385.74. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006; 16:R551–60. PMID: 16860735.75. Madeo F, Carmona-Gutierrez D, Kepp O, Kroemer G. Spermidine delays aging in humans. Aging (Albany NY). 2018; 10:2209–2211. PMID: 30082504.76. Elhassan YS, Kluckova K, Fletcher RS, et al. Nicotinamide riboside augments the human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures in aged subjects: a placebo-controlled, randomized trial. bioRxiv. 2019; 680462.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mitochondrial quality control and its emerging role in the pathogenesis of diabetic kidney disease
- Mitochondrial Gene Therapy
- Therapeutic Modulation of Apoptosis: Targeting the BCL-2 Family at the Interface of the Mitochondrial Membrane
- Positioning Metabolism as a Central Player in the Diabetic Heart
- Post-Translational Modifications of Cardiac Mitochondrial Proteins in Cardiovascular Disease: Not Lost in Translation