Endocrinol Metab.
2020 Mar;35(1):14-25. 10.3803/EnM.2020.35.1.14.
Unmet Clinical Needs in the Treatment of Patients with Thyroid Cancer
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kimwb@amc.seoul.kr
- KMID: 2471716
- DOI: http://doi.org/10.3803/EnM.2020.35.1.14
Abstract
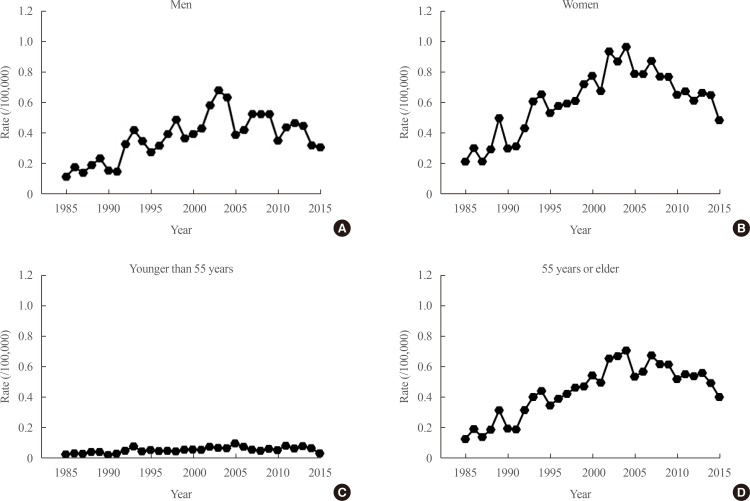
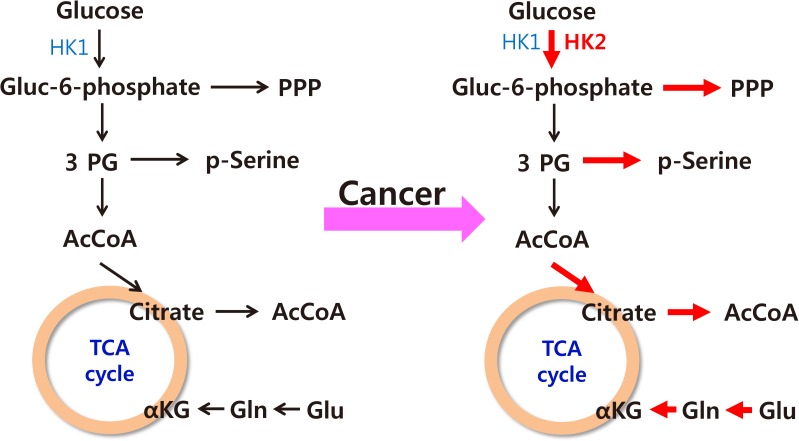
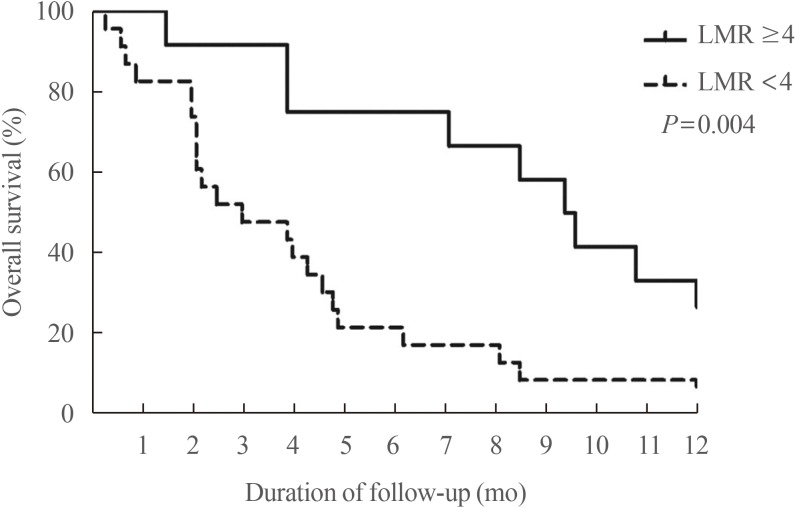
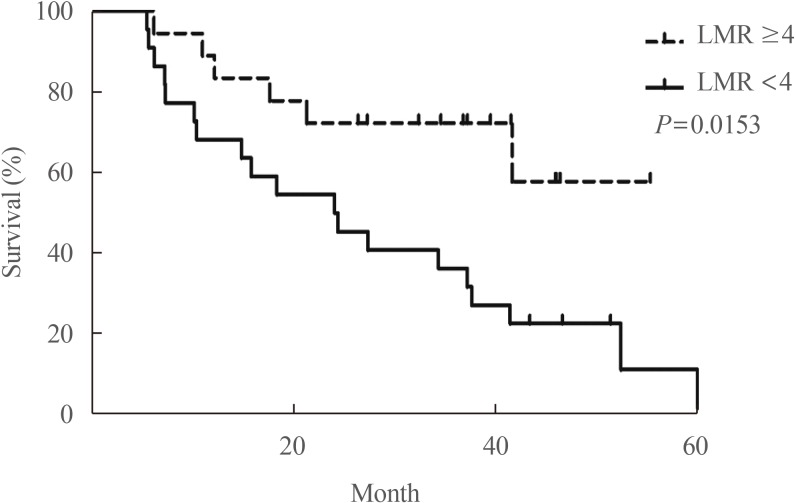
- The increased incidence of thyroid cancer is a worldwide phenomenon; however, the issue of overdiagnosis has been most prominent in South Korea. The age-standardized mortality rate of thyroid cancer in Korea steeply increased from 1985 to 2004 (from 0.17 per 100,000 to 0.85 per 100,000), and then decreased until 2015 to 0.42 per 100,000, suggesting that early detection reduced mortality. However, early detection of thyroid cancer may be cost-ineffective, considering its very high prevalence and indolent course. Therefore, risk stratification and tailored management are vitally important, but many prognostic markers can only be evaluated postoperatively. Discovery of preoperative marker(s), especially for small cancers, is the most important unmet clinical need for thyroid cancer. Herein, we discuss some such factors that we recently discovered. Another unmet clinical need is better treatment of radioiodine-refractory (RAIR) differentiated thyroid cancer (DTC) and undifferentiated cancers. Although sorafenib and lenvatinib are available, better drugs are needed. We found that phosphoglycerate dehydrogenase, a critical enzyme for serine biosynthesis, could be a novel therapeutic target, and that the lymphocyte-to-monocyte ratio is a prognostic marker of survival in patients with anaplastic thyroid carcinoma or RAIR DTC. Deeper insights are needed into tumor-host interactions in thyroid cancer to improve treatment.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Transcriptomic Analysis of Papillary Thyroid Cancer: A Focus on Immune-Subtyping, Oncogenic Fusion, and Recurrence
Seung-Jin Park, Yea Eun Kang, Jeong-Hwan Kim, Jong-Lyul Park, Seon-Kyu Kim, Seung-Woo Baek, In Sun Chu, Shinae Yi, Seong Eun Lee, Young Joo Park, Eun-Jae Chung, Jin Man Kim, Hye Mi Ko, Je-Ryong Kim, Seung-Nam Jung, Ho-Ryun Won, Jae Won Chang, Bon Seok Koo, Seon-Young Kim
Clin Exp Otorhinolaryngol. 2022;15(2):183-193. doi: 10.21053/ceo.2021.02215.Lactate Dehydrogenase A as a Potential New Biomarker for Thyroid Cancer
Eun Jeong Ban, Daham Kim, Jin Kyong Kim, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Kee-Hyun Nam, Woong Youn Chung, Kunhong Kim
Endocrinol Metab. 2021;36(1):96-105. doi: 10.3803/EnM.2020.819.
Reference
-
1. Burman KD, Wartofsky L. Thyroid nodules. N Engl J Med. 2016; 374:1294–1295.
Article2. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994; 154:1838–1840. PMID: 8053752.
Article3. Leenhardt L, Bernier MO, Boin-Pineau MH, Conte Devolx B, Marechaud R, Niccoli-Sire P, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004; 150:133–139. PMID: 14763910.
Article4. Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The Impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid. 2015; 25:999–1007. PMID: 26103159.
Article5. Aschebrook-Kilfoy B, Schechter RB, Shih YC, Kaplan EL, Chiu BC, Angelos P, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013; 22:1252–1259. PMID: 23677575.
Article6. Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996; 156:2165–2172. PMID: 8885814.
Article7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.
Article8. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372:621–630. PMID: 25671254.
Article9. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384:319–328. PMID: 24768112.
Article10. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009; 20:525–531. PMID: 19016336.
Article11. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006; 295:2164–2167. PMID: 16684987.
Article12. Sosa JA, Hanna JW, Robinson KA, Lanman RB. Increases in thyroid nodule fine-needle aspirations, operations, and diagnoses of thyroid cancer in the United States. Surgery. 2013; 154:1420–1426. PMID: 24094448.
Article13. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017; 317:1338–1348. PMID: 28362912.
Article14. Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015; 16:1042–1054. PMID: 26365757.
Article15. Kitahara CM, Linet MS, Beane Freeman LE, Check DP, Church TR, Park Y, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control. 2012; 23:1615–1624. PMID: 22843022.
Article16. Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. Thyroid cancer in Denmark 1943-2008, before and after iodine supplementation. Int J Cancer. 2012; 131:2360–2366. PMID: 22337133.
Article17. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013; 2013:965212. PMID: 23737785.
Article18. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015; 136:2187–2195. PMID: 25284703.
Article19. Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2016; 27:316–322. PMID: 26441345.20. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016; 375:614–617. PMID: 27532827.
Article21. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141. PMID: 25761484.
Article22. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–430. PMID: 30913865.
Article23. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014; 371:1765–1767. PMID: 25372084.24. Ahn HS, Kim HJ, Kim KH, Lee YS, Han SJ, Kim Y, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016; 26:1535–1540. PMID: 27627550.
Article25. Park S, Oh CM, Cho H, Lee JY, Jung KW, Jun JK, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016; 355:i5745. PMID: 27903497.
Article26. Choi YM, Kim WG, Kwon H, Jeon MJ, Han M, Kim TY, et al. Changes in standardized mortality rates from thyroid cancer in Korea between 1985 and 2015: analysis of Korean national data. Cancer. 2017; 123:4808–4814. PMID: 28817188.
Article27. Han JM, Kim TY, Jeon MJ, Yim JH, Kim WG, Song DE, et al. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol. 2013; 168:879–886. PMID: 23513231.
Article28. Kim WG, Kim WB, Woo G, Kim H, Cho Y, Kim TY, et al. Thyroid stimulating hormone reference range and prevalence of thyroid dysfunction in the Korean population: Korea National Health and Nutrition Examination Survey 2013 to 2015. Endocrinol Metab (Seoul). 2017; 32:106–114. PMID: 28116874.
Article29. Kim WG, Kim EY, Yim JH, Han JM, Jeon MJ, Kim TY, et al. Comparison of different staging systems for predicting recurrence of papillary thyroid carcinoma. Endocrinol Metab (Seoul). 2011; 26:53–61.
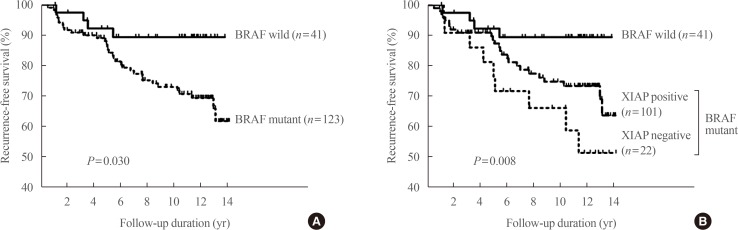
Article30. Chung KW, Yang SK, Lee GK, Kim EY, Kwon S, Lee SH, et al. Detection of BRAFV600E mutation on fine needle aspiration specimens of thyroid nodule refines cyto-pathology diagnosis, especially in BRAF600E mutation-prevalent area. Clin Endocrinol (Oxf). 2006; 65:660–666. PMID: 17054470.31. Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2006; 65:364–368. PMID: 16918957.
Article32. Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 2017; 3:202–208. PMID: 27581851.33. Xing M. Genetic-guided risk assessment and management of thyroid cancer. Endocrinol Metab Clin North Am. 2019; 48:109–124. PMID: 30717896.
Article34. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016; 23:R143–R155. PMID: 26733501.
Article35. Jeon MJ, Chun SM, Lee JY, Choi KW, Kim D, Kim TY, et al. Mutational profile of papillary thyroid microcarcinoma with extensive lymph node metastasis. Endocrine. 2019; 64:130–138. PMID: 30645724.
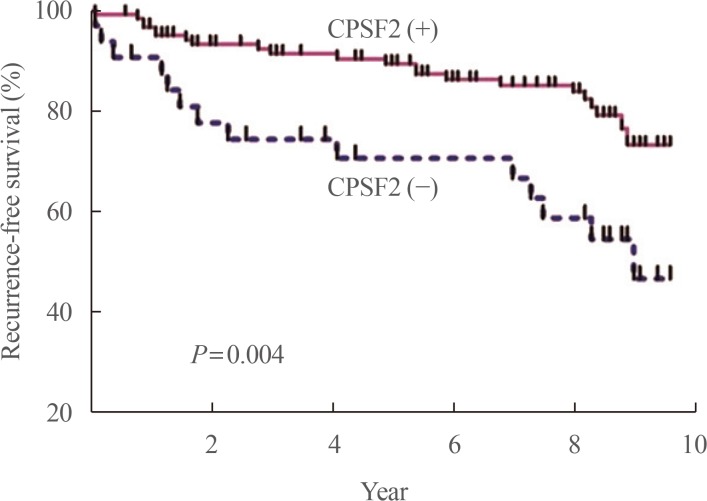
Article36. Yim JH, Kim WG, Jeon MJ, Han JM, Kim TY, Yoon JH, et al. Association between expression of X-linked inhibitor of apoptosis protein and the clinical outcome in a BRAF V600E-prevalent papillary thyroid cancer population. Thyroid. 2014; 24:689–694. PMID: 24124924.37. Sung TY, Kim M, Kim TY, Kim WG, Park Y, Song DE, et al. Negative expression of CPSF2 predicts a poorer clinical outcome in patients with papillary thyroid carcinoma. Thyroid. 2015; 25:1020–1025. PMID: 26148673.
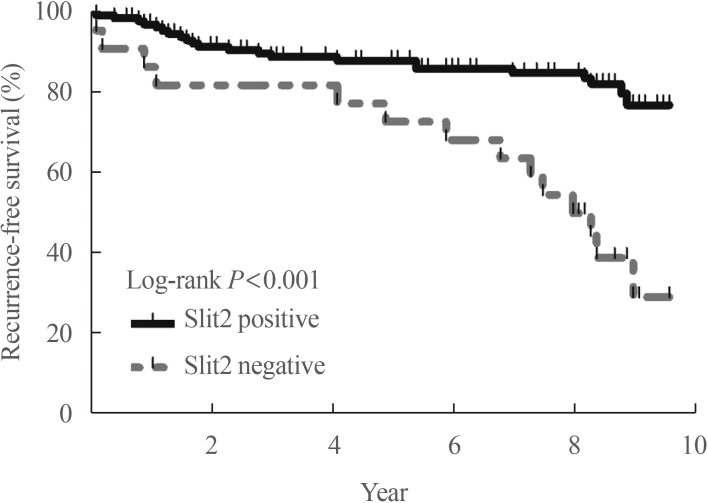
Article38. Jeon MJ, Lim S, You MH, Park Y, Song DE, Sim S, et al. The role of Slit2 as a tumor suppressor in thyroid cancer. Mol Cell Endocrinol. 2019; 483:87–96. PMID: 30648543.
Article39. Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012; 491:399–405. PMID: 23103869.40. He H, Bronisz A, Liyanarachchi S, Nagy R, Li W, Huang Y, et al. SRGAP1 is a candidate gene for papillary thyroid carcinoma susceptibility. J Clin Endocrinol Metab. 2013; 98:E973–E980. PMID: 23539728.41. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006; 91:2892–2899. PMID: 16684830.
Article42. Hamilton SN, Tran E, Berthelet E, Wu J. The role of external beam radiation therapy in well-differentiated thyroid cancer. Expert Rev Anticancer Ther. 2017; 17:905–910. PMID: 28750593.
Article43. Dunne EM, Fraser IM, Liu M. Stereotactic body radiation therapy for lung, spine and oligometastatic disease: current evidence and future directions. Ann Transl Med. 2018; 6:283. PMID: 30105233.
Article44. Choy PY, Koea J, McCall J, Holden A, Osbourne M. The role of radiofrequency ablation in the treatment of primary and metastatic tumours of the liver: initial lessons learned. N Z Med J. 2002; 115:U128. PMID: 12362172.45. Mazzeo S, Cervelli R, Elisei R, Tarantini G, Cappelli C, Molinaro E, et al. mRECIST criteria to assess recurrent thyroid carcinoma treatment response after radiofrequency ablation: a prospective study. J Endocrinol Invest. 2018; 41:1389–1399. PMID: 29687416.
Article46. Hay ID, Lee RA, Davidge-Pitts C, Reading CC, Charboneau JW. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. 2013; 154:1448–1454. PMID: 24176579.47. Ribechini A, Bottici V, Chella A, Elisei R, Vitti P, Pinchera A, et al. Interventional bronchoscopy in the treatment of tracheal obstruction secondary to advanced thyroid cancer. J Endocrinol Invest. 2006; 29:131–135. PMID: 16610238.
Article48. Deschamps F, de Baere T. Cementoplasty of bone metastases. Diagn Interv Imaging. 2012; 93:685–689. PMID: 22889809.
Article49. Moneke I, Kaifi JT, Kloeser R, Samson P, Haager B, Wiesemann S, et al. Pulmonary metastasectomy for thyroid cancer as salvage therapy for radioactive iodine-refractory metastases. Eur J Cardiothorac Surg. 2018; 53:625–630. PMID: 29092022.
Article50. Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019; 19:196. PMID: 30832606.
Article51. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018; 36:7–13. PMID: 29072975.
Article52. Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012; 12:487–493. PMID: 22695393.
Article53. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674. PMID: 21376230.
Article54. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011; 11:85–95. PMID: 21258394.
Article55. Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Control. 2000; 7:229–239. PMID: 10832109.
Article56. Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009; 14:216–221. PMID: 19270027.
Article57. Lee KH, Seok EY, Kim EY, Yun JS, Park YL, Park CH. Different prognostic values of individual hematologic parameters in papillary thyroid cancer due to age-related changes in immunity. Ann Surg Treat Res. 2019; 96:70–77. PMID: 30746354.
Article58. Ahn J, Song E, Oh HS, Song DE, Kim WG, Kim TY, et al. Low lymphocyte-to-monocyte ratios are associated with poor overall survival in anaplastic thyroid carcinoma patients. Thyroid. 2019; 29:824–829. PMID: 30864902.
Article59. Ahn J, Song E, Kim WG, Kim TY, Kim WB, Shong YK, et al. Prognostic role of the lymphocyte-to-monocyte ratio for clinical outcomes of patients with progressive radioiodine-refractory differentiated thyroid carcinoma treated by sorafenib. Clin Endocrinol (Oxf). 2020; 92:71–76. PMID: 31663136.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Literature Review on Unmet Needs of High-Prevalence Cancer Survivors: Focus on Breast Cancer, Thyroid Cancer, Colorectal Cancer, and Lung Cancer
- Unmet Needs and Sexual Distress of Gynecological Cancer Patients according to the Period after Initial Treatment
- Incidence and clinical characteristics of thyroid cancer in Korea
- Unmet Needs and Quality of Life of Colorectal Cancer Survivors Immediately after Treatment Ends and 5 Years
- Giant Thyroid Cancer in Elderly Patient