Korean J Radiol.
2020 Feb;21(2):210-217. 10.3348/kjr.2019.0557.
In Vivo Detection of Lipid-Core Plaques by Coronary CT Angiography: A Head-to-Head Comparison with Histologic Findings
- Affiliations
-
- 1Department of Radiology, Fuwai Hospital, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. blu@vip.sina.com
- 2Department of Radiology, Affiliated Hospital of Guizhou Medical University, Guiyang, China.
- 3Department of Pathology, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
- 4Department of Cardiac Surgery, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
- KMID: 2471638
- DOI: http://doi.org/10.3348/kjr.2019.0557
Abstract
OBJECTIVE
We sought to distinguish lipid plaques using a CT quantitative pixel density histogram, based on the pathological diagnosis of lipid cores as the gold standard.
MATERIALS AND METHODS
Eight patients awaiting heart transplantation due to end-stage coronary heart disease underwent coronary CT angiography (CCTA) spectroscopy prior to heart transplantation; coronary artery pathological analysis was performed for all patients. Lipid-core plaques were defined pathologically as manifesting a lipid core diameter > 200 µm, a circumference > 60 degrees, and a cap thickness < 450 µm. The percentage distributions of CT pixel attenuation ≤ 20, 30, 40, and 50 HU were calculated using quantitative histogram analysis.
RESULTS
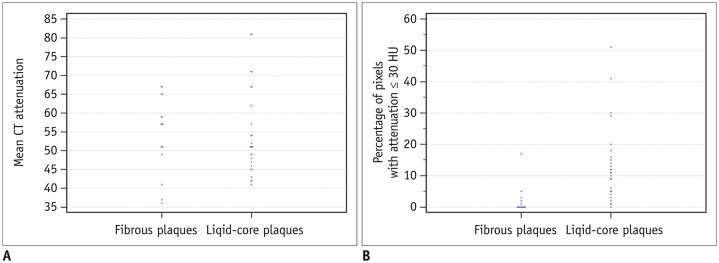
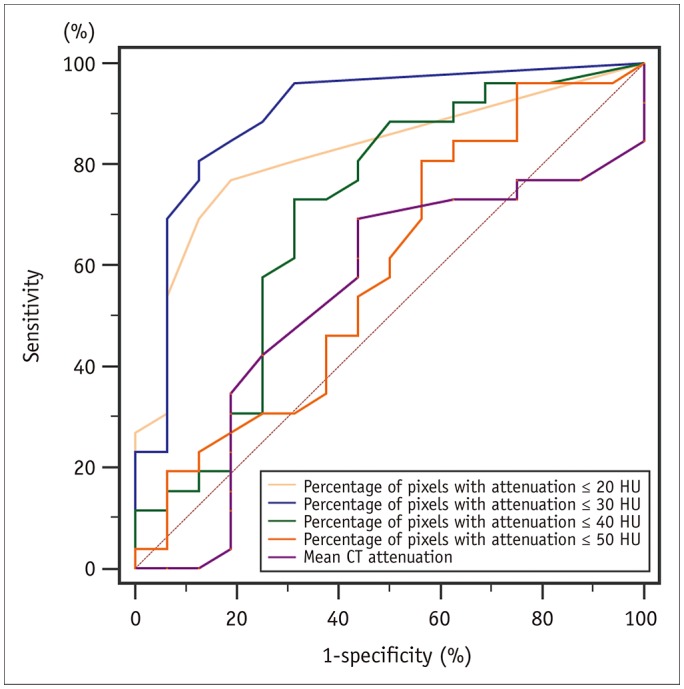
A total of 271 transverse sections were co-registered between CCTA and pathological analysis. Overall, 26 lipid cores and 16 fibrous plaques were identified by pathological analysis. There was no significant difference in median CT attenuation between the lipid and fibrous plaques (51 HU [interquartile range, 46-63] vs. 57 HU [interquartile range, 50-64], p = 0.659). The median percentage of CT pixel attenuation ≤ 30 HU accounted for 11% (5-17) of lipid-core plaques and 0% (0-2) of fibrous plaques (p < 0.001). The sensitivity and specificity of the method for diagnosing lipid plaques by the average CT pixel attenuation ≤ 30 HU were 80.8% and 87.5%, respectively. The area under the receiver operator characteristics curve was 0.898 (95% confidence interval: 0.765-0.970; 3.0% was the best cut-off value). The diagnostic performance was significantly higher than those of the average pixel CT attenuation percentages ≤ 20, 40, and 50 HU and the mean CT attenuation (p < 0.05).
CONCLUSION
In in vivo conditions, with the pathological lipid core as the gold standard, quantification of the percentage of average CT pixel attenuation ≤ 30 HU in the histogram can be useful for accurate identification of lipid plaques.
MeSH Terms
Figure
Reference
-
1. Chang HJ, Lin FY, Lee SE, Andreini D, Bax J, Cademartiri F, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018; 71:2511–2522. PMID: 29852975.
Article2. Chow BJ, Wells GA, Chen L, Yam Y, Galiwango P, Abraham A, et al. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol. 2010; 55:1017–1028. PMID: 20202518.3. Ferencik M, Schlett CL, Ghoshhajra BB, Kriegel MF, Joshi SB, Maurovich-Horvat P, et al. A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol. 2012; 110:183–189. PMID: 22481015.
Article4. Pohle K, Achenbach S, Macneill B, Ropers D, Ferencik M, Moselewski F, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis. 2007; 190:174–180. PMID: 16494883.
Article5. Leschka S, Seitun S, Dettmer M, Baumüller S, Stolzmann P, Goetti R, et al. Ex vivo evaluation of coronary atherosclerotic plaques: characterization with dual-source CT in comparison with histopathology. J Cardiovasc Comput Tomogr. 2010; 4:301–308. PMID: 20947041.
Article6. Schlett CL, Maurovich-Horvat P, Ferencik M, Alkadhi H, Stolzmann P, Scheffel H, et al. Histogram analysis of lipid-core plaques in coronary computed tomographic angiography: ex vivo validation against histology. Invest Radiol. 2013; 48:646–653. PMID: 23614976.7. Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhbäck A, et al. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011; 215:110–115. PMID: 21227419.
Article8. Fuchs TA, Stehli J, Fiechter M, Dougoud S, Gebhard C, Ghadri JR, et al. First experience with monochromatic coronary computed tomography angiography from a 64-slice CT scanner with gemstone spectral imaging (GSI). J Cardiovasc Comput Tomogr. 2013; 7:25–31. PMID: 23452997.
Article9. Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008; 1:638–648. PMID: 19356494.
Article10. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.
Article11. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009; 54:49–57. PMID: 19555840.
Article12. Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N, et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017; 18:772–779. PMID: 27502292.13. Leber AW, Knez A, Becker A, Becker C, von Ziegler F, Nikolaou K, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004; 43:1241–1247. PMID: 15063437.14. Petranovic M, Soni A, Bezzera H, Loureiro R, Sarwar A, Raffel C, et al. Assessment of nonstenotic coronary lesions by 64-slice multidetector computed tomography in comparison to intravascular ultrasound: evaluation of nonculprit coronary lesions. J Cardiovasc Comput Tomogr. 2009; 3:24–31. PMID: 19201374.
Article15. Schroeder S, Kopp AF, Baumbach A, Meisner C, Kuettner A, Georg C, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001; 37:1430–1435. PMID: 11300457.16. Dalager MG, Bøttcher M, Dalager S, Andersen G, Thygesen J, Pedersen EM, et al. Imaging atherosclerotic plaques by cardiac computed tomography in vitro: impact of contrast type and acquisition protocol. Invest Radiol. 2011; 46:790–795. PMID: 21826008.17. Dalager MG, Bøttcher M, Andersen G, Thygesen J, Pedersen EM, Dejbjerg L, et al. Impact of luminal density on plaque classification by CT coronary angiography. Int J Cardiovasc Imaging. 2011; 27:593–600. PMID: 20820922.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Noninvasive Detection of Coronary Atherosclerotic Plaques and Assessment of Stenosis Degree at Multidetector CT Coronary Angiography
- An Overview of Myocardial Bridging With a Focus on Multidetector CT Coronary Angiographic Findings
- Evaluation of Atherosclerotic Plaque in Patients without Coronary Artery Calcification Using Multidetector Row Computed Tomography: A Preliminary Report of 110 patients
- Optimizing the Imaging Protocol for Ex Vivo Coronary Artery Wall Using High-Resolution MRI: An Experimental Study on Porcine and Human
- Diagnostic accuracy of 64-slice multi-detector CT coronary angiography in the evaluation of coronary artery disease