Korean J Radiol.
2020 Feb;21(2):146-158. 10.3348/kjr.2019.0500.
Ultrasonographic Demonstration of the Tissue Microvasculature in Children: Microvascular Ultrasonography Versus Conventional Color Doppler Ultrasonography
- Affiliations
-
- 1Department of Radiology, Korea University Hospital, Ansan, Korea. radje@korea.ac.kr
- 2K Eye Clinic, Hongseong, Korea.
- KMID: 2471632
- DOI: http://doi.org/10.3348/kjr.2019.0500
Abstract
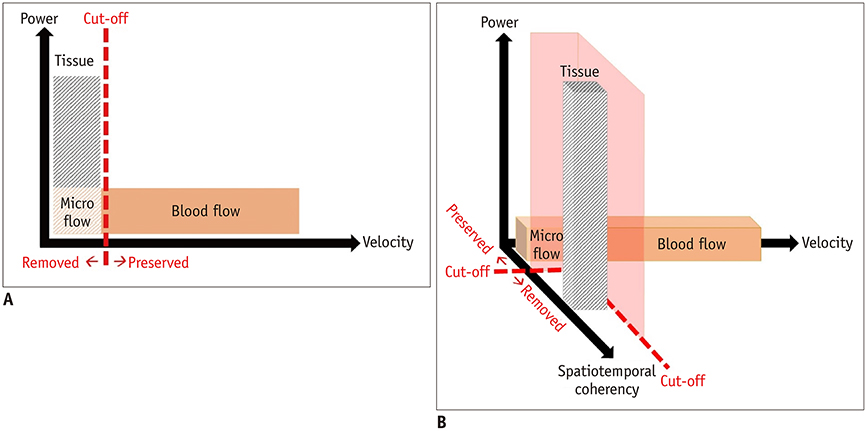
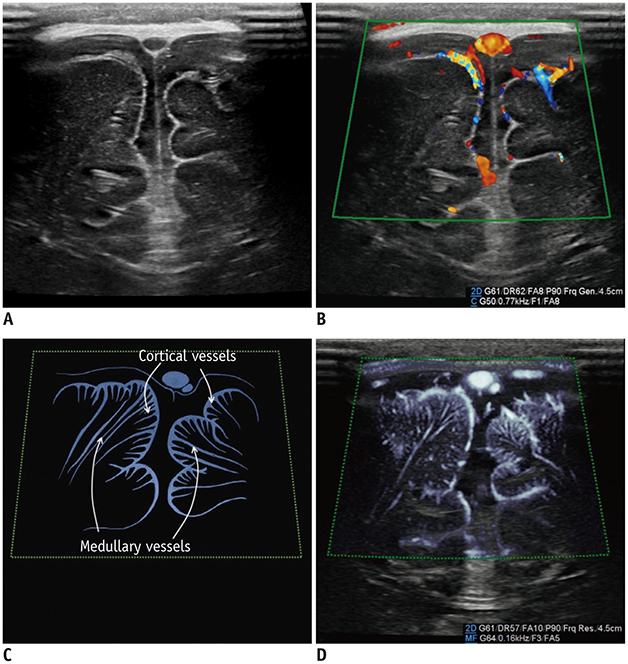
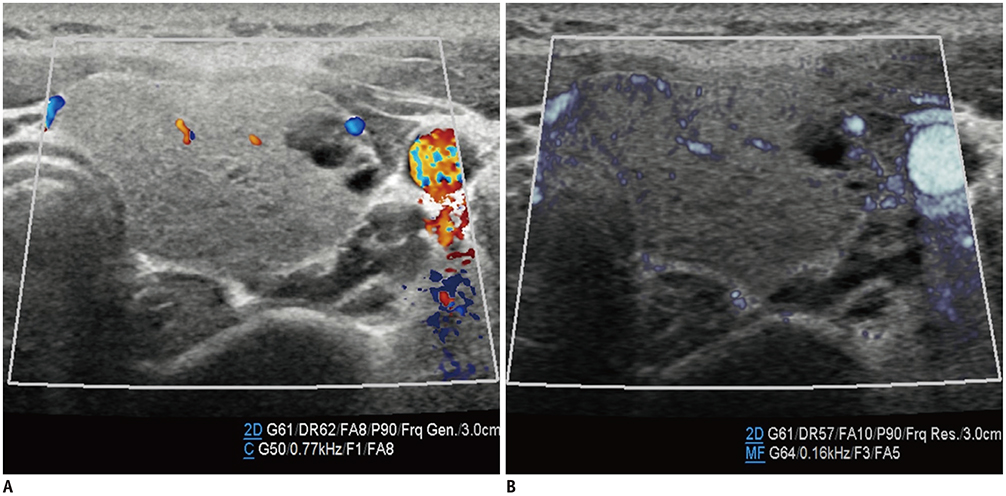
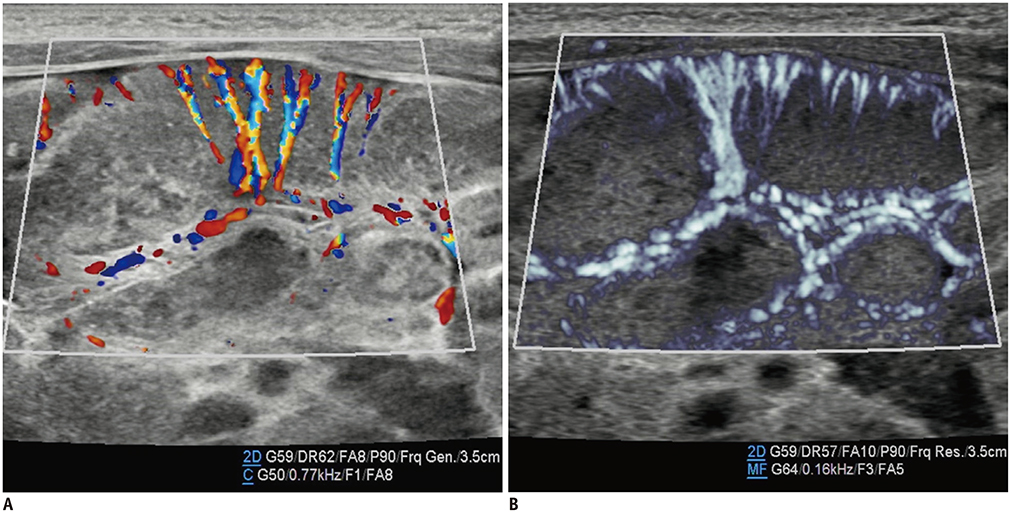
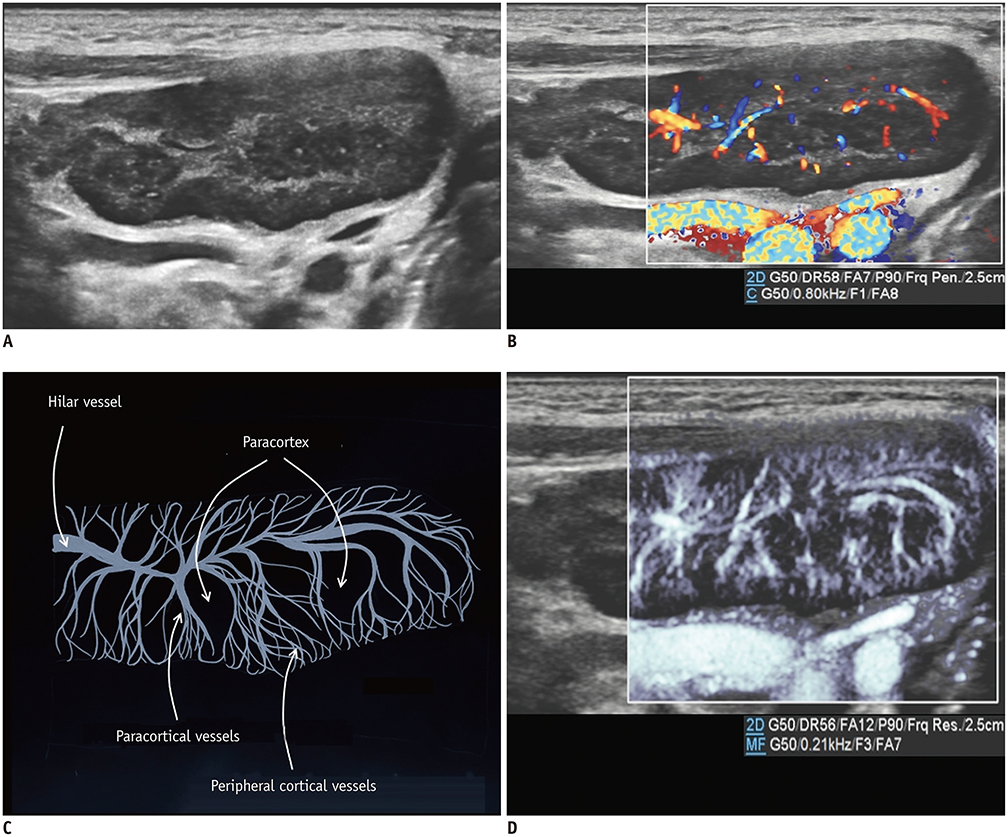
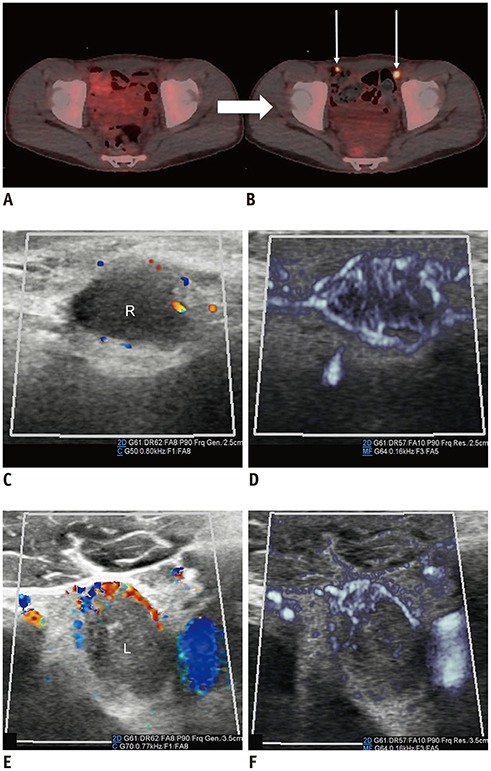
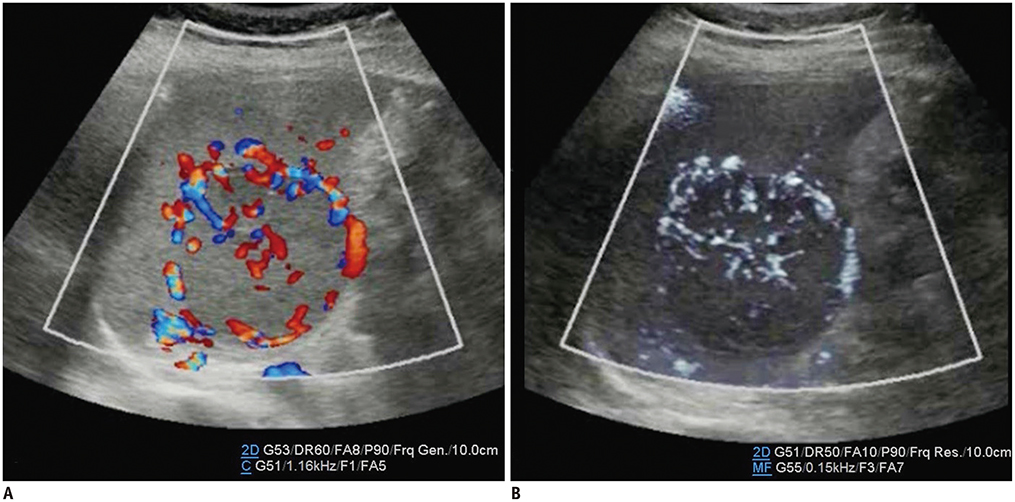
- Microvascular ultrasonographic imaging is the most recent and unique Doppler ultrasound technique. It uses an advanced clutter filter that can remove clutter artifacts and preserve the low-velocity microvascular flow signal. The potential advantages of microvascular ultrasonography are its superiority in detection and visualization of the small blood vessels in tissues, providing radiologists with more information on the vascular structures. Therefore, it has shown particular value in the clinical fields. The aim of this study was to provide microvascular ultrasonographic images for the tissue microvasculature, including the brain, thyroid gland, kidney, urinary bladder, small bowel, ovary, testis, lymph node, and hemangiomas in children, focusing on the comparison with conventional color Doppler ultrasonographic images.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Usefulness of Real-Time Quantitative Microvascular Ultrasonography for Differentiation of Graves’ Disease from Destructive Thyroiditis in Thyrotoxic Patients
Han-Sang Baek, Ji-Yeon Park, Chai-Ho Jeong, Jeonghoon Ha, Moo Il Kang, Dong-Jun Lim
Endocrinol Metab. 2022;37(2):323-332. doi: 10.3803/EnM.2022.1413.
Reference
-
1. Babcock DS, Patriquin H, LaFortune M, Dauzat M. Power doppler sonography: basic principles and clinical applications in children. Pediatr Radiol. 1996; 26:109–115.
Article2. Park AY, Seo BK. Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography. 2018; 37:98–106.
Article3. Goeral K, Hojreh A, Kasprian G, Klebermass-Schrehof K, Weber M, Mitter C, et al. Microvessel ultrasound of neonatal brain parenchyma: feasibility, reproducibility, and normal imaging features by superb microvascular imaging (SMI). Eur Radiol. 2019; 29:2127–2136.
Article4. Ayaz E, Aslan A, İnan İ, Yıkılmaz A. Evaluation of ovarian vascularity in children by using the “superb microvascular imaging” ultrasound technique in comparison with conventional Doppler ultrasound techniques. J Ultrasound Med. 2019; 38:2751–2760.
Article5. Kim HK, O'Hara S, Je BK, Kraus SJ, Horn P. Feasibility of superb microvascular imaging to detect high-grade vesicoureteral reflux in children with urinary tract infection. Eur Radiol. 2018; 28:66–73.
Article6. Ohno Y, Fujimoto T, Shibata Y. A new era in diagnostic ultrasound, superb microvascular imaging: preliminary results in pediatric hepato-gastrointestinal disorders. Eur J Pediatr Surg. 2017; 27:20–25.
Article7. Lee YS, Kim MJ, Han SW, Lee HS, Im YJ, Shin HJ, et al. Superb microvascular imaging for the detection of parenchymal perfusion in normal and undescended testes in young children. Eur J Radiol. 2016; 85:649–656.
Article8. Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. 2nd ed. Wien: Springer-Verlag;1999. p. 429–458.9. Jiang ZZ, Huang YH, Shen HL, Liu XT. Clinical applications of superb microvascular imaging in the liver, breast, thyroid, skeletal muscle, and carotid plaques. J Ultrasound Med. 2019; 38:2811–2820.
Article10. Machado P, Segal S, Lyshchik A, Forsberg F. A novel microvascular flow technique: initial results in thyroids. Ultrasound Q. 2016; 32:67–74.11. Chen L, Zhan J, Diao XH, Liu YC, Shi YX, Chen Y, et al. Additional value of superb microvascular imaging for thyroid nodule classification with the thyroid imaging reporting and data system. Ultrasound Med Biol. 2019; 45:2040–2048.
Article12. Bayramoglu Z, Kandemirli SG, Caliskan E, Yilmaz R, Kardelen AD, Poyrazoglu S, et al. Assessment of paediatric Hashimoto's thyroiditis using superb microvascular imaging. Clin Radiol. 2018; 73:1059.e9–1059.e15.
Article13. Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013; 3:817–831.
Article14. Kim B, Lim HK, Choi MH, Woo JY, Ryu J, Kim S, et al. Detection of parenchymal abnormalities in acute pyelonephritis by pulse inversion harmonic imaging with or without microbubble ultrasonographic contrast agent: correlation with computed tomography. J Ultrasound Med. 2001; 20:5–14.
Article15. Craig WD, Wagner BJ, Travis MD. Pyelonephritis: radiologic-pathologic review. Radiographics. 2008; 28:255–277.
Article16. Eisberg HB. Intestinal arteries. Anat Rec. 1924; 28:227–242.
Article17. Clement PB. Histology of the ovary. Am J Surg Pathol. 1987; 11:277–303.
Article18. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015; 21:56–83.
Article19. Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006; 34:409–424.
Article20. Ahuja A, Ying M. Sonography of neck lymph nodes. Part II: abnormal lymph nodes. Clin Radiol. 2003; 58:359–366.
Article21. Darrow DH, Greene AK, Mancini AJ, Nopper AJ. Section on Dermatology, Section on Otolaryngology-Head and Neck Surgery, And Section on Plastic Surgery. Diagnosis and management of infantile hemangioma: executive summary. Pediatrics. 2015; 136:786–791.
Article22. Fishman SJ, Mulliken JB. Hemangiomas and vascular malformations of infancy and childhood. Pediatr Clin North Am. 1993; 40:1177–1200.
Article23. Weber FC, Greene AK, Adams DM, Liang MG, Alomari MH, Voss SD, et al. Role of imaging in the diagnosis of parotid infantile hemangiomas. Int J Pediatr Otorhinolaryngol. 2017; 102:61–66.
Article24. Vancauwenberghe T, Snoeckx A, Vanbeckevoort D, Dymarkowski S, Vanhoenacker FM. Imaging of the spleen: what the clinician needs to know. Singapore Med J. 2015; 56:133–144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Ultrasonographic Demonstration of the Tissue Microvasculature in Children: Microvascular Ultrasonography Versus Conventional Color Doppler Ultrasonography
- Diagnostic Value of Color Doppler Ultrasonography of UPJ Obstruction
- General principles of carotid Doppler ultrasonography
- Doppler ultrasonography of the lower extremity arteries: anatomy and scanning guidelines
- Differentiations of Retinal Detachment and Vitreous Membrane Using Color Doppler Imaging