Korean Circ J.
2020 Apr;50(4):317-327. 10.4070/kcj.2019.0258.
Safety and Efficacy of a New Ultrathin Sirolimus-Eluting Stent with Abluminal Biodegradable Polymer in Real-World Practice
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea. jyoon@yonsei.ac.kr
- 2Division of Cardiology, Department of Internal Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- 3Department of Cardiology, Cardiovascular Center, Korea University Anam Hospital, Korea University, Seoul, Korea.
- 4Division of Cardiology, Department of Internal Medicine, Konkuk University Chungju Hospital, Konkuk University College of Medicine, Chungju, Korea.
- 5Division of Cardiovascular Medicine, Department of Internal Medicine, Dankook University Hospital, Dankook University College of Medicine, Cheonan, Korea.
- 6Division of Cardiology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 7Division of Cardiology, Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- 8Division of Cardiology, Department of Internal Medicine, Kangnam Sacred Heart Hospital, Hallym University, Seoul, Korea.
- 9Division of Cardiology, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University, Goyang, Korea.
- 10Department of Cardiology, Gachon University Gil Medical Center, Gachon University, Incheon, Korea.
- 11Division of Cardiology, Department of Internal Medicine, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 12Division of Cardiology, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 13Department of Internal Medicine, Kangwon National University Hospital, Kangwon National University, Chuncheon, Korea.
- 14Division of cardiology, Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University, Busan, Korea.
- 15Division of Cardiology, Heart Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 16Division of Cardiology, Cardiovascular Center, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2471281
- DOI: http://doi.org/10.4070/kcj.2019.0258
Abstract
- BACKGROUND AND OBJECTIVES
Recently, Genoss drug-eluting stent (DES)â„¢ stent comprising cobalt-chromium platform with an ultrathin strut thickness, sirolimus, and an abluminal biodegradable polymer was developed. Owing to the lack of substantial evidence for the safety and efficacy of this stent, we report 12-month results of the Genoss DESâ„¢ stent.
METHODS
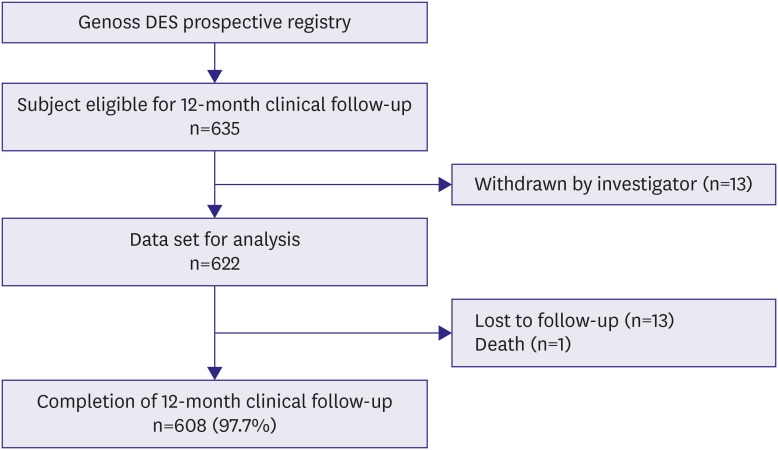
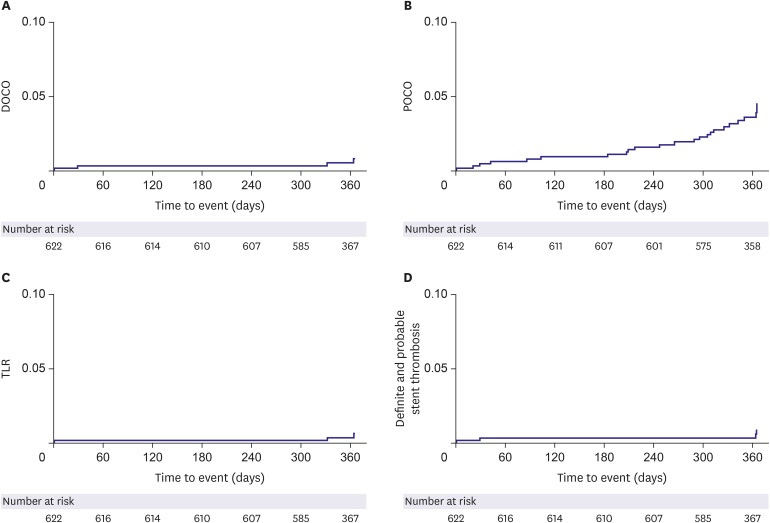
We analyzed subjects who were eligible for a 12-month follow-up from the ongoing Genoss DESâ„¢ registry, which is a prospective, single-arm, observational, multicenter trial to investigate the clinical outcomes after the successful Genoss DESâ„¢ stent implantation among all-comers. The primary endpoint was a device-oriented composite outcome, defined as cardiac death, target vessel-related myocardial infarction, and target lesion revascularization at 12-month follow-up.
RESULTS
Among 622 subjects, the mean age of subjects was 66.5±10.4 years, 70.6% were males, 67.5% had hypertension, and 38.3% had diabetes. The implanted stent number, diameter, and length per patient were 1.5±0.8, 3.1±0.4 mm, and 36.0±23.3 mm, respectively. At 12-month clinical follow-up, the primary endpoint occurred only in 4 (0.6%) subjects.
CONCLUSIONS
The novel Genoss DESâ„¢ stent exhibited excellent safety and efficacy in real-world practice.
MeSH Terms
Figure
Cited by 2 articles
-
Can Genoss DES™ Stand Out in the Crowd of Stents?
Jae-Hwan Lee, Jae-Hyeong Park
Korean Circ J. 2020;50(4):328-329. doi: 10.4070/kcj.2020.0040.State-of-the-Art Stent Technology to Minimize the Risk of Stent Thrombosis and In-Stent Restenosis: Abluminal-Coated Biodegradable Polymer Drug-Eluting Stent
Dong Oh Kang, Cheol Ung Choi
Korean Circ J. 2022;52(5):365-367. doi: 10.4070/kcj.2022.0017.
Reference
-
1. Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005; 294:1215–1223. PMID: 16160130.2. Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003; 349:1315–1323. PMID: 14523139.3. Holmes DR Jr, Kereiakes DJ, Laskey WK, et al. Thrombosis and drug-eluting stents: an objective appraisal. J Am Coll Cardiol. 2007; 50:109–118. PMID: 17616294.4. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356:998–1008. PMID: 17296824.5. El-Hayek G, Bangalore S, Casso Dominguez A, et al. Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc Interv. 2017; 10:462–473. PMID: 28279314.6. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014; 35:1147–1158. PMID: 24459196.7. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014; 63:299–307. PMID: 24211507.8. Yang HM, Seo KW, Yoon J, et al. Clinical and angiographic outcomes of the first Korean-made sirolimus-eluting coronary stent with abluminal bioresorbable polymer. Korean Circ J. 2017; 47:898–906. PMID: 29035435.9. Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016; 68:1116–1139. PMID: 27036919.10. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018; 39:213–260. PMID: 28886622.11. Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007; 115:2344–2351. PMID: 17470709.12. Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation. 2018; 138:2216–2226. PMID: 29945934.13. Zbinden R, Piccolo R, Heg D, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable-polymer everolimus-eluting stent for percutaneous coronary revascularization: 2-year results of the BIOSCIENCE Trial. J Am Heart Assoc. 2016; 5:e003255. PMID: 26979080.14. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007; 357:2001–2015. PMID: 17982182.15. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–1057. PMID: 19717846.16. Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011; 377:1409–1420. PMID: 21470671.17. Levine GN, Jeong YH, Goto S, et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014; 11:597–606. PMID: 25154978.18. Kang J, Kim HS. The evolving concept of dual antiplatelet therapy after percutaneous coronary intervention: focus on unique feature of East Asian and “Asian Paradox”. Korean Circ J. 2018; 48:537–551. PMID: 29968428.19. Kok MM, Zocca P, Buiten RA, et al. Two-year clinical outcome of all-comers treated with three highly dissimilar contemporary coronary drug-eluting stents in the randomised BIO-RESORT trial. EuroIntervention. 2018; 14:915–923. PMID: 29790480.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Very Late Stent Thrombosis Related to Fracture of a Sirolimus-Eluting Stent
- Coronary Stent Thrombosis: Current Insights into New Drug-Eluting Stent Designs
- A Case of Late Recurrent Vasospasm After Sirolimus-Eluting Stent Implantation
- A Case of Stent Thrombosis Occurred at 5 Years after Sirolimus-Eluting Stent Implantation
- Drug-Eluting Stent Strut Fracture as a Cause of Restenosis