Korean Circ J.
2020 Apr;50(4):302-316. 10.4070/kcj.2019.0291.
Percutaneous Pulmonary Valve Implantation
- Affiliations
-
- 1Department of Pediatric and Adult Congenital Cardiology and Cardiac Surgery, IRCCS Policlinico San Donato, San Donato Milanese, Italy. Mario.Carminati@grupposandonato.it
- KMID: 2471280
- DOI: http://doi.org/10.4070/kcj.2019.0291
Abstract
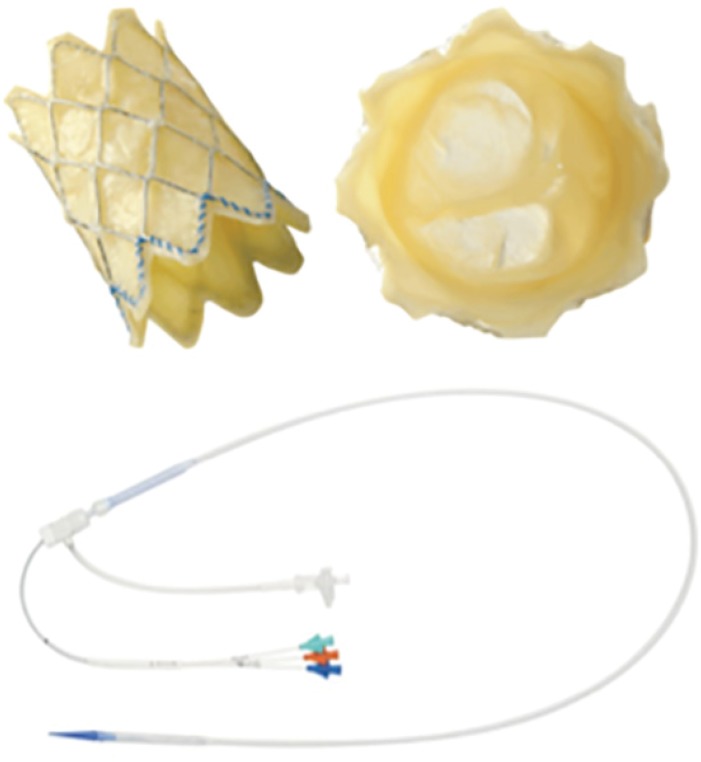
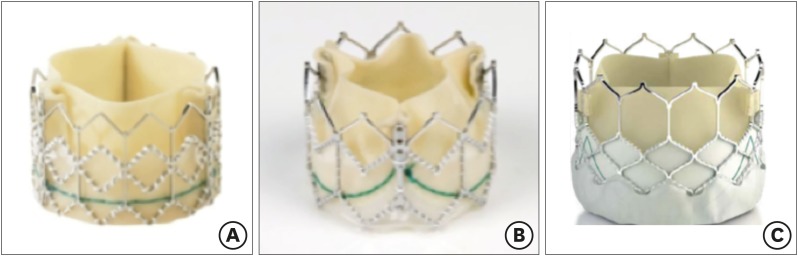
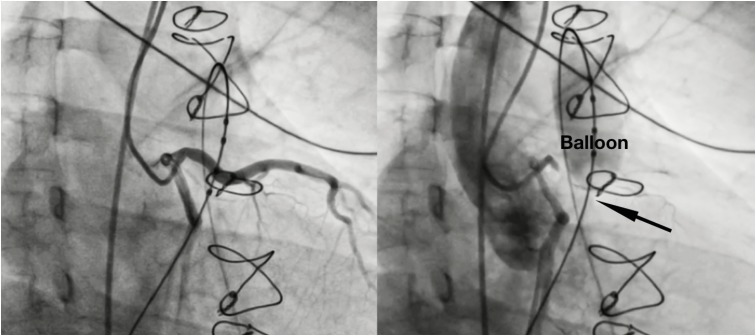
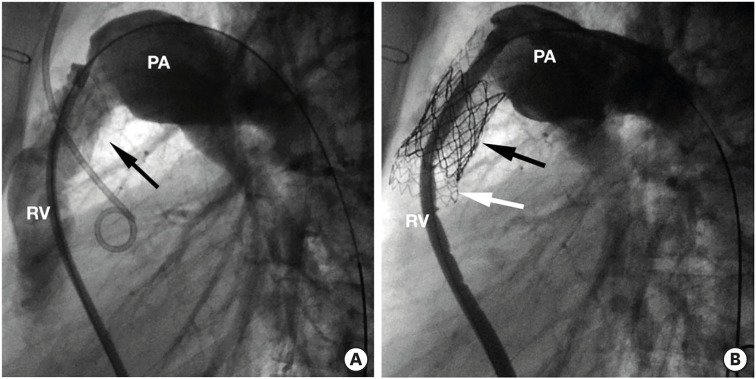
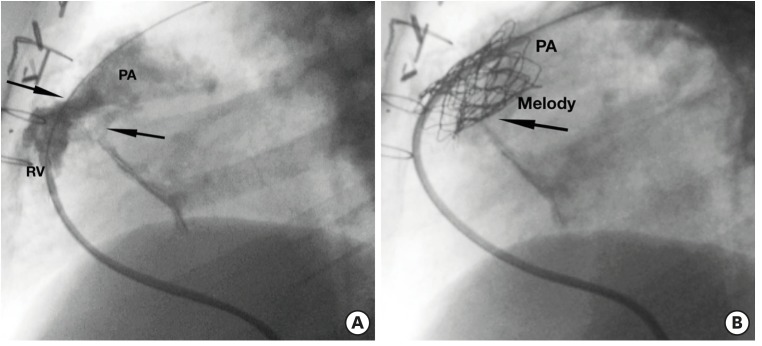
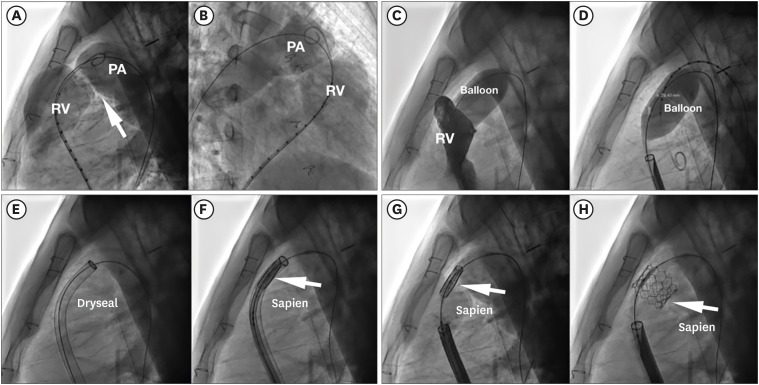
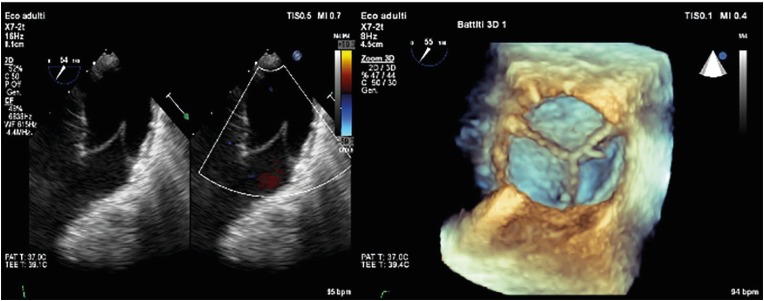
- Percutaneous pulmonary valve implantation (PPVI) is recognized as a feasible and low risk alternative to surgery to treat dysfunctional right ventricular outflow tract (RVOT) in usually pluri-operated patients. Evolving technology allowed to develop different kind of prosthesis and to go from an initial treatment exclusively of stenotic conduit to an actual approach extended also to wide native RVOT. The Melody transcatheter pulmonary valve (TPV) and the Edwards Sapien valve are nowadays the most commonly implanted prostheses. However, other devices have been developed to treat large RVOT (i.e., the Venus p-valve, the Medtronic Harmony TPV, the Alterra Adaptive Prestent, and the Pulsta valve). Indications for PPVI are the same as for surgical interventions on pulmonary valve, with limits related to the maximum diameter of the available percutaneous prosthesis. Therefore, an accurate preoperative evaluation is of paramount importance to select patients who could benefit from this procedure. The overall periprocedural mortality incidence is around 1.4%, while freedom from RVOT reintervention ranges from 100% at 4 months to 70% at 70 months, according to the different published studies.
Keyword
MeSH Terms
Figure
Reference
-
1. Vricella LA, Kanani M, Cook AC, Cameron DE, Tsang VT. Problems with the right ventricular outflow tract: a review of morphologic features and current therapeutic options. Cardiol Young. 2004; 14:533–549. PMID: 15680076.2. Biernacka EK, Rużyłło W, Demkow M. Percutaneous pulmonary valve implantation - state of the art and Polish experience. Postepy Kardiol Interwencyjnej. 2017; 13:3–9. PMID: 28344611.3. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019; 139:e637–97. PMID: 30586768.4. Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010; 31:2915–2957. PMID: 20801927.5. Silversides CK, Marelli A, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: executive summary. Can J Cardiol. 2010; 26:143–150. PMID: 20352134.6. Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation. 2000; 102:813–816. PMID: 10942752.7. Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000; 356:1403–1405. PMID: 11052583.8. Bonhoeffer P, Boudjemline Y, Qureshi SA, et al. Percutaneous insertion of the pulmonary valve. J Am Coll Cardiol. 2002; 39:1664–1669. PMID: 12020495.9. Qureshi AM, Prieto LR. Percutaneous pulmonary valve placement. Tex Heart Inst J. 2015; 42:195–201. PMID: 26175629.10. Boshoff DE, Cools BL, Heying R, et al. Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv. 2013; 81:987–995. PMID: 22887796.11. Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011; 58:2248–2256. PMID: 22078433.12. Suntharos P, Prieto LR. Percutaneous pulmonary valve implantation in the native right ventricular outflow tract using a 29-mm Edwards SAPIEN 3 valve. World J Pediatr Congenit Heart Surg. 2017; 8:639–642. PMID: 27864470.13. Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert Rev Cardiovasc Ther. 2009; 7:823–833. PMID: 19589118.14. Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009; 10:1–25. PMID: 19065003.15. Lancellotti P, Tribouilloy C, Hagendorff A, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr. 2010; 11:223–244. PMID: 20375260.16. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23:685–713. PMID: 20620859.17. Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006; 81:1603–1611. PMID: 17165639.18. Miliaresis C, Beker S, Gewitz M. Cardiopulmonary stress testing in children and adults with congenital heart disease. Cardiol Rev. 2014; 22:275–278. PMID: 25162333.19. Sanz J, Conroy J, Narula J. Imaging of the right ventricle. Cardiol Clin. 2012; 30:189–203. PMID: 22548811.20. Tezza M, Witsenburg M, Nieman K, van de Woestijne PC, Budde RP. Cardiac CT to assess the risk of coronary compression in patients evaluated for percutaneous pulmonary valve implantation. Eur J Radiol. 2019; 110:88–96. PMID: 30599879.21. Meier LM, Meineri M, Qua Hiansen J, Horlick EM. Structural and congenital heart disease interventions: the role of three-dimensional printing. Neth Heart J. 2017; 25:65–75. PMID: 28083857.22. Vukicevic M, Mosadegh B, Min JK, Little SH. Cardiac 3D printing and its future directions. JACC Cardiovasc Imaging. 2017; 10:171–184. PMID: 28183437.23. Chessa M, Giugno L, Butera G, Carminati M. Multi-modal imaging support in a staging percutaneous pulmonary valve implantation. Eur Heart J. 2016; 37:66. PMID: 26056126.24. McElhinney DB, Hennesen JT. The Melody® valve and Ensemble® delivery system for transcatheter pulmonary valve replacement. Ann N Y Acad Sci. 2013; 1291:77–85. PMID: 23834411.25. Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol. 2009; 53:1855–1858. PMID: 19442884.26. Binder RK, Rodés-Cabau J, Wood DA, et al. Transcatheter aortic valve replacement with the SAPIEN 3: a new balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv. 2013; 6:293–300. PMID: 23517842.27. Aldoss O, Fonseca BM, Truong UT, et al. Diagnostic utility of three-dimensional rotational angiography in congenital cardiac catheterization. Pediatr Cardiol. 2016; 37:1211–1221. PMID: 27278632.28. Stenger A, Dittrich S, Glöckler M. Three-dimensional rotational angiography in the pediatric cath lab: optimizing aortic interventions. Pediatr Cardiol. 2016; 37:528–536. PMID: 26667957.29. Manica JL, Borges MS, Medeiros RF, Fischer LS, Broetto G, Rossi Filho RI. A comparison of radiation dose between standard and 3D angiography in congenital heart disease. Arq Bras Cardiol. 2014; 103:131–137. PMID: 25211313.30. Nguyen HH, Balzer DT, Murphy JJ, Nicolas R, Shahanavaz S. Radiation exposure by three-dimensional rotational angiography (3DRA) during trans-catheter melody pulmonary valve procedures (TMPV) in a pediatric cardiac catheterization laboratory. Pediatr Cardiol. 2016; 37:1429–1435. PMID: 27452802.31. Goreczny S, Moszura T, Dryzek P, et al. Three-dimensional image fusion guidance of percutaneous pulmonary valve implantation to reduce radiation exposure and contrast dose: a comparison with traditional two-dimensional and three-dimensional rotational angiographic guidance. Neth Heart J. 2017; 25:91–99. PMID: 27966187.32. Ansari MM, Cardoso R, Garcia D, et al. Percutaneous pulmonary valve implantation: present status and evolving future. J Am Coll Cardiol. 2015; 66:2246–2255. PMID: 26564602.33. Malekzadeh-Milani S, Ladouceur M, Cohen S, Iserin L, Boudjemline Y. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2014; 107:592–598. PMID: 25218009.34. Shivaraju A, Kodali S, Thilo C, et al. Overexpansion of the SAPIEN 3 transcatheter heart valve: a feasibility study. JACC Cardiovasc Interv. 2015; 8:2041–2043. PMID: 26738676.35. Hascoet S, Karsenty C, Tortigue M, et al. A modified procedure for percutaneous pulmonary valve implantation of the Edwards SAPIEN 3 valve. EuroIntervention. 2019; 14:1386–1388. PMID: 30327285.36. Hascoët S, Acar P, Boudjemline Y. Transcatheter pulmonary valvulation: current indications and available devices. Arch Cardiovasc Dis. 2014; 107:625–634. PMID: 25444020.37. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter pulmonary valve replacement with the Edwards Sapien system. The Toronto experience. JACC Cardiovasc Interv. 2015; 8:1819–1827. PMID: 26718514.38. Morray BH, McElhinney DB, Cheatham JP, et al. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013; 6:535–542. PMID: 24065444.39. Lindsay I, Aboulhosn J, Salem M, Levi D. Aortic root compression during transcatheter pulmonary valve replacement. Catheter Cardiovasc Interv. 2016; 88:814–821. PMID: 27121036.40. Faccini A, Butera G. Tricuspid regurgitation as a complication of Edwards Sapien XT valve implantation in pulmonary position a problem to deal with. Catheter Cardiovasc Interv. 2018; 91:927–931. PMID: 29405557.41. Virk SA, Liou K, Chandrakumar D, Gupta S, Cao C. Percutaneous pulmonary valve implantation: a systematic review of clinical outcomes. Int J Cardiol. 2015; 201:487–489. PMID: 26313872.42. Lurz P, Nordmeyer J, Giardini A, et al. Early versus late functional outcome after successful percutaneous pulmonary valve implantation: are the acute effects of altered right ventricular loading all we can expect? J Am Coll Cardiol. 2011; 57:724–731. PMID: 21292132.43. Jones TK, Rome JJ, Armstrong AK, et al. Transcatheter pulmonary valve replacement reduces tricuspid regurgitation in patients with right ventricular volume/pressure overload. J Am Coll Cardiol. 2016; 68:1525–1535. PMID: 27687194.44. Borik S, Crean A, Horlick E, et al. Percutaneous pulmonary valve implantation: 5 years of follow-up: does age influence outcomes? Circ Cardiovasc Interv. 2015; 8:e001745. PMID: 25652317.45. Pagourelias ED, Daraban AM, Mada RO, et al. Right ventricular remodelling after transcatheter pulmonary valve implantation. Catheter Cardiovasc Interv. 2017; 90:407–417. PMID: 28296032.46. Garay F, Pan X, Zhang YJ, Wang C, Springmuller D. Early experience with the Venus p‑valve for percutaneous pulmonary valve implantation in native outflow tract. Neth Heart J. 2017; 25:76–81. PMID: 27943178.47. Promphan W, Prachasilchai P, Siripornpitak S, Qureshi SA, Layangool T. Percutaneous pulmonary valve implantation with the Venus P-valve: clinical experience and early results. Cardiol Young. 2016; 26:698–710. PMID: 26088820.48. Schoonbeek RC, Takebayashi S, Aoki C, et al. Implantation of the Medtronic Harmony transcatheter pulmonary valve improves right ventricular size and function in an ovine model of postoperative chronic pulmonary insufficiency. Circ Cardiovasc Interv. 2016; 9:e003920. PMID: 27662847.49. Zahn EM, Chang JC, Armer D, Garg R. First human implant of the Alterra Adaptive PrestentTM: a new self-expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018; 91:1125–1129. PMID: 29521437.50. Kim GB, Song MK, Bae EJ, et al. Successful feasibility human trial of a new self-expandable percutaneous pulmonary valve (Pulsta Valve) implantation using knitted nitinol wire backbone and trileaflet α-gal-free porcine pericardial valve in the native right ventricular outflow tract. Circ Cardiovasc Interv. 2018; 11:e006494. PMID: 29871940.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous Pulmonary Valve Implantation
- Percutaneous transluminal balloon valvuloplasty for congenital pulmonary valve stenosis and aortic valve stenosis
- Percutaneous Transluminal Balloon Valvuloplasty for Congenital Pulmonary Valve Stenosis
- Successful emergency transcatheter aortic valve implantation
- Echocardiography in Transcatheter Aortic Valve Implantation and Mitral Valve Clip