Ann Surg Treat Res.
2020 Mar;98(3):130-138. 10.4174/astr.2020.98.3.130.
Management of isolated para-aortic lymph node recurrence after surgery for colorectal cancer
- Affiliations
-
- 1Department of Colon and Rectal Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ipark@amc.seoul.kr
- 2Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 2471178
- DOI: http://doi.org/10.4174/astr.2020.98.3.130
Abstract
- PURPOSE
The rare incidence of isolated para-aortic lymph node (PALN) recurrence of colorectal cancer has precluded the formulation of treatment guidelines. This study evaluated and compared the effects of different treatment modalities on survival outcomes in patients with PALN recurrence.
METHODS
Patients diagnosed with isolated PALN recurrence after curative resection for primary colorectal cancer from January 2004 to December 2014 were evaluated retrospectively. Patients with isolated recurrence were selected using imaging modalities. Overall survival (OS) and survival after recurrence (SAR) were analyzed and compared between different treatments using the Kaplan-Meier method.
RESULTS
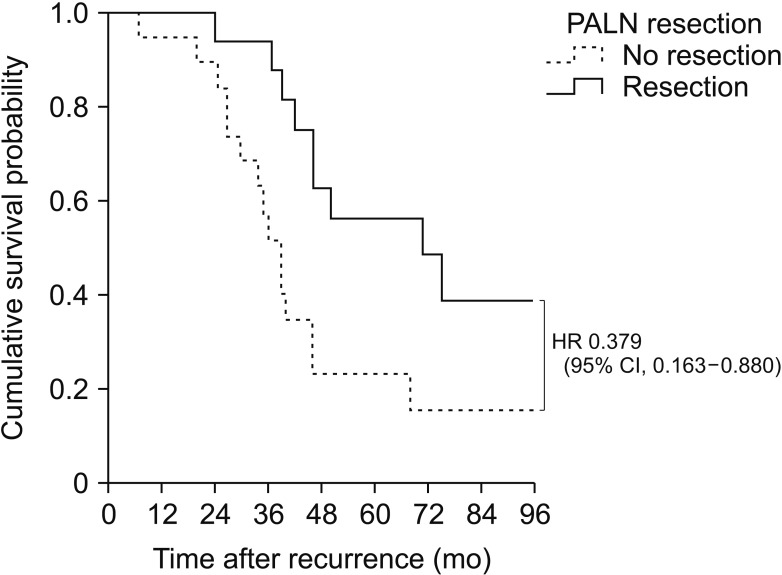
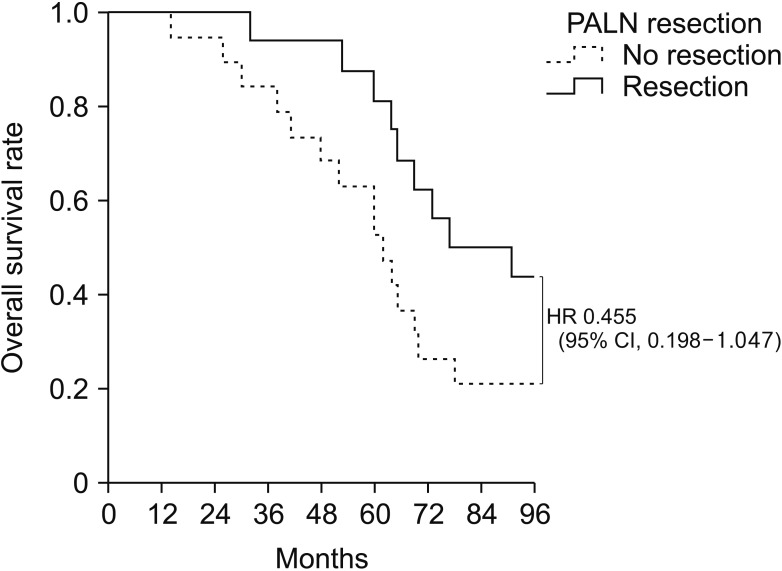
The median OS was 64 months with a median follow-up time of 50 months. Of the 46 patients with PALN recurrence, 35 (76.1%) had isolated recurrences. Of these 35 patients, 16 underwent PALN resection and 19 received chemotherapy. Median SAR was significantly longer in patients who did than did not undergo resection (71 months vs. 39 months, P = 0.017). Median OS tended to be longer in patients who did than did not undergo resection (77 months vs. 62 months, P = 0.055). SAR was similar in patients who received radiotherapy and those who underwent resection (34 months vs. 46 months, P = 0.146). Three of 16 patients (18.8%) who underwent resection were found to be recurrence-free.
CONCLUSION
Surgical resection of isolated PALN recurrence may benefit patients, with favorable survival outcomes and by providing definitive diagnosis for proper treatment planning.
MeSH Terms
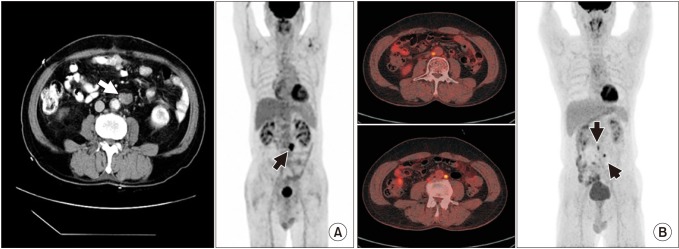
Figure
Reference
-
1. Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J Surg Oncol. 2008; 97:136–140. PMID: 17963247.
Article2. Bellier J, De Wolf J, Hebbar M, Amrani ME, Desauw C, Leteurtre E, et al. Repeated resections of hepatic and pulmonary metastases from colorectal cancer provide long-term survival. World J Surg. 2018; 42:1171–1179. PMID: 28948336.
Article3. Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007; 84:324–338. PMID: 17588454.
Article4. Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F, et al. The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis. J Surg Oncol. 2016; 114:803–809. PMID: 27792291.
Article5. Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006; 94:982–999. PMID: 16538219.
Article6. Klement RJ, Abbasi-Senger N, Adebahr S, Alheid H, Allgaeuer M, Becker G, et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer. 2019; 19:173. PMID: 30808323.
Article7. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008; 371:1007–1016. PMID: 18358928.
Article8. Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017; 109(9):DOI: 10.1093/jnci/djx015.
Article9. Lee S, Kim DY, Kim SY, Koom WS, Lee SY, Kang JK, et al. Curative radiotherapy using different radiation techniques for isolated lung metastasis from colorectal cancer. Tumori. 2013; 99:68–75. PMID: 23549003.
Article10. D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011; 18:1096–1103. PMID: 21042942.11. Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003; 50:1362–1366. PMID: 14571738.12. Selman TJ, Mann C, Zamora J, Appleyard TL, Khan K. Diagnostic accuracy of tests for lymph node status in primary cervical cancer: a systematic review and meta-analysis. CMAJ. 2008; 178:855–862. PMID: 18362381.
Article13. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004; 232:773–783. PMID: 15273331.
Article14. Kennedy ED, Milot L, Fruitman M, Al-Sukhni E, Heine G, Schmocker S, et al. Development and implementation of a synoptic MRI report for preoperative staging of rectal cancer on a population-based level. Dis Colon Rectum. 2014; 57:700–708. PMID: 24807594.
Article15. Schulz A, Viktil E, Godt JC, Johansen CK, Dormagen JB, Holtedahl JE, et al. Diagnostic performance of CT, MRI and PET/CT in patients with suspected colorectal liver metastases: the superiority of MRI. Acta Radiol. 2016; 57:1040–1048. PMID: 26622057.
Article16. Sivesgaard K, Larsen LP, Sorensen M, Kramer S, Schlander S, Amanavicius N, et al. Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol. 2018; 28:4735–4747. PMID: 29736846.
Article17. Taylor SA, Mallett S, Beare S, Bhatnagar G, Blunt D, Boavida P, et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed colorectal cancer: the prospective Streamline C trial. Lancet Gastroenterol Hepatol. 2019; 4:529–537. PMID: 31080095.18. Takeshima K, Yamafuji K, Asami A, Baba H, Okamoto N, Takahashi H, et al. Successful resection of isolated paraaortic lymph node recurrence from advanced sigmoid colon cancer following 156 courses of FOLFIRI regimen. Case Rep Surg. 2016; 2016:4548798. PMID: 27648336.
Article19. Yeo SG, Kim DY, Kim TH, Jung KH, Hong YS, Kim SY, et al. Curative chemoradiotherapy for isolated retroperitoneal lymph node recurrence of colorectal cancer. Radiother Oncol. 2010; 97:307–311. PMID: 20667611.
Article20. Shibata D, Paty PB, Guillem JG, Wong WD, Cohen AM. Surgical management of isolated retroperitoneal recurrences of colorectal carcinoma. Dis Colon Rectum. 2002; 45:795–801. PMID: 12072633.
Article21. Bae SH, Kim MS, Cho CK, Kang JK, Kang HJ, Kim YH, et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol. 2012; 106:138–143. PMID: 22297789.
Article22. Filippi AR, Guerrera F, Badellino S, Ceccarelli M, Castiglione A, Guarneri A, et al. Exploratory analysis on overall survival after either surgery or stereotactic radiotherapy for lung oligometastases from colorectal cancer. Clin Oncol (R Coll Radiol). 2016; 28:505–512. PMID: 26899780.
Article23. He X, Zhang P, Li Z, Bi F, Xu F, Wang X, et al. Curative-intent radiotherapy in patients with oligometastatic lesions from colorectal cancer: a single-center study. Medicine (Baltimore). 2018; 97:e12601. PMID: 30290630.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laparoscopic para-aortic lymph node dissection for patients with primary colorectal cancer and clinically suspected para-aortic lymph nodes
- Laparoscopy-assisted gastrectomy with para-aortic lymphadenectomy after palliative chemotherapy for advanced gastric cancer with isolated para-aortic lymph node metastasis
- Prognosis of the Patients Showing Metastasis to the Para-aortic or/and Supraclavicular Lymph Nodes at the Time of Diagnosis of Recurrence of the Cervical Cancer
- Para-aortic Lymph Node Dissection in Gastric Cancer
- Laparoscopic Para-aortic Lymph Node Dissection in Patients with Gynecologic Malignancy