Korean J Physiol Pharmacol.
2020 Mar;24(2):157-163. 10.4196/kjpp.2020.24.2.157.
Eupatilin downregulates phorbol 12-myristate 13-acetate-induced MUC5AC expression via inhibition of p38/ERK/JNK MAPKs signal pathway in human airway epithelial cells
- Affiliations
-
- 1Center for Core Research Facilities, Wonkwang University School of Medicine, Iksan 54538, Korea.
- 2Department of Physiology, Wonkwang University School of Medicine, Iksan 54538, Korea.
- 3Medical Convergence Research Center, Wonkwang University Hospital, Iksan 54538, Korea.
- 4Department of Otolaryngology, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan 54538, Korea. Leejaehoon64@gmail.com
- KMID: 2471037
- DOI: http://doi.org/10.4196/kjpp.2020.24.2.157
Abstract
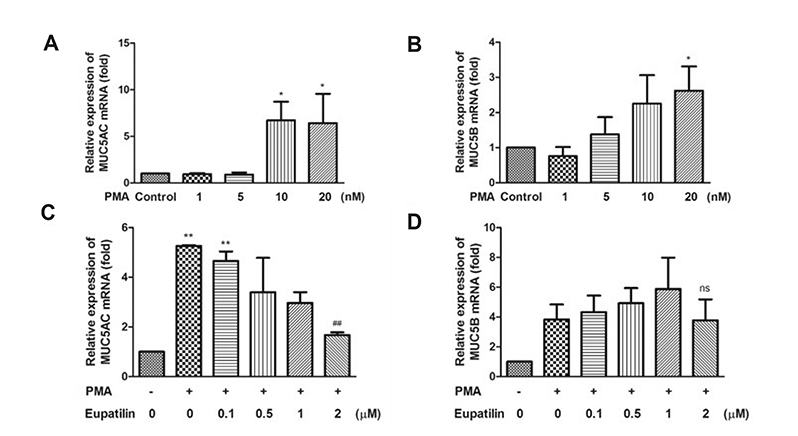
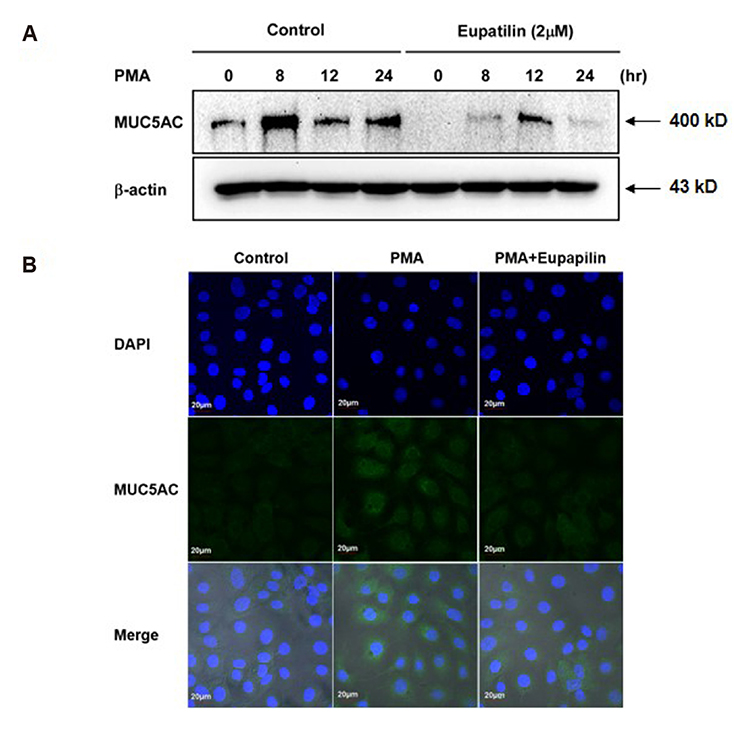
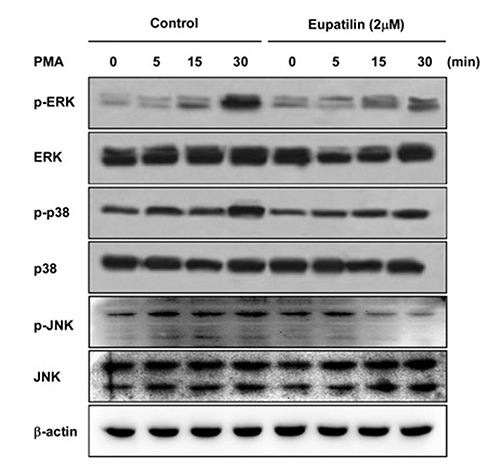
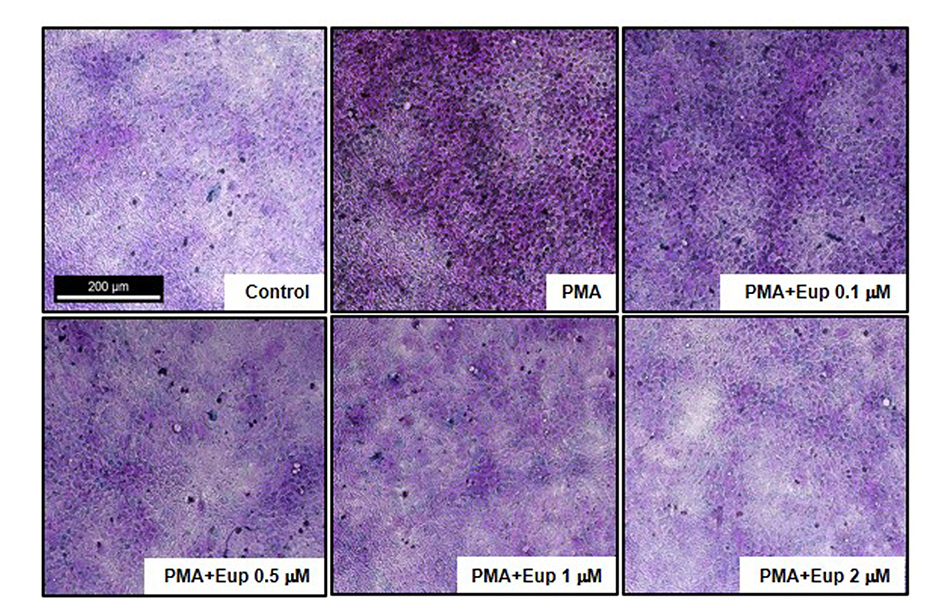
- Chronic inflammatory airway diseases, such as chronic rhinosinusitis, chronic obstructive pulmonary disease, and asthma, are associated with excessive mucus production. Hence, the regulation of mucus production is important for the treatment of upper and lower airway diseases. Eupatilin is a pharmacologically active ingredient obtained from Artemisia asiatica Nakai (Asteraceae) and exerts potent anti-inflammatory, anti-allergic, and anti-tumor activities. In the present study, we investigated the effect of eupatilin on phorbol 12-myristate 13-acetate (PMA)-induced MUC5AC and MUC5B expression in human airway epithelial cells. We found that eupatilin treatment significantly inhibited PMA-induced mucus secretion in PAS staining. In addition, qRT-PCR results showed that eupatilin dose-dependently decreased the mRNA expression of MUC5AC in human airway epithelial cells. Western blot and immunofluorescence assay also showed that PMA-induced protein expression of MUC5AC was inhibited by eupatilin treatment. Finally, we investigated MAPKs activity after stimulation with PMA using western blot analysis in human airway epithelial cells. The results showed that eupatilin downregulated the levels of phosphorylated p38, ERK, and JNK. In summary, the anti-inflammatory activities of eupatilin, characterized as the suppression of MUC5AC expression and secretion in human airway epithelial cells, were found to be associated with the inhibition of p38/ERK/JNK MAPKs signaling pathway of MUC5AC secretion.
MeSH Terms
Figure
Reference
-
1. Bae CH, Jeon BS, Choi YS, Song SY, Kim YD. Delphinidin inhibits LPS-induced MUC8 and MUC5B expression through toll-like receptor 4-mediated ERK1/2 and p38 MAPK in human airway epithelial cells. Clin Exp Otorhinolaryngol. 2014; 7:198–204.
Article2. Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992; 101:916–921.
Article3. Ali Mel-S. Nasosinus mucin expression in normal and inflammatory conditions. Curr Opin Allergy Clin Immunol. 2009; 9:10–15.
Article4. Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002; 122:6 Suppl. 320S–326S.
Article5. Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009; 135:505–512.
Article6. Jung J, Ko SH, Yoo do Y, Lee JY, Kim YJ, Choi SM, Kang KK, Yoon HJ, Kim H, Youn J, Kim JM. 5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 via the Akt and nuclear factor-κB-dependent pathway, leading to suppression of adhesion of monocytes and eosinophils to bronchial epithelial cells. Immunology. 2012; 137:98–113.7. Kim DH, Na HK, Oh TY, Kim WB, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces cell cycle arrest in ras-transformed human mammary epithelial cells. Biochem Pharmacol. 2004; 68:1081–1087.
Article8. Seo HJ, Park KK, Han SS, Chung WY, Son MW, Kim WB, Surh YJ. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. Int J Cancer. 2002; 100:456–462.9. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004; 344:683–695.10. Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol. 2007; 170:20–32.
Article11. Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, Lee CJ. Phorbol ester or epidermal growth-factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012; 26:1784–1788.
Article12. Park SH, Lee HJ, Ryu J, Son KH, Kwon SY, Lee SK, Kim YS, Hong JH, Seok JH, Lee CJ. Effects of ophiopogonin D and spicatoside A derived from Liriope Tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine. 2014; 21:172–176.
Article13. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.14. Takeyama K, Dabbagh K, Lee HM, Agustí C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.
Article15. Fischer BM, Rochelle LG, Voynow JA, Akley NJ, Adler KB. Tumor necrosis factor-alpha stimulates mucin secretion and cyclic GMP production by guinea pig tracheal epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999; 20:413–422.16. Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, Yoon JH. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003; 278:23243–23250.17. Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003; 221:42–49.
Article18. Park SJ, Kang SY, Kim NS, Kim HM. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol Immunotoxicol. 2002; 24:211–226.
Article19. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125.
Article20. Song EH, Chung KS, Kang YM, Lee JH, Lee M, An HJ. Eupatilin suppresses the allergic inflammatory response in vitro and in vivo. Phytomedicine. 2018; 42:1–8.21. Jeon JI, Ko SH, Kim YJ, Choi SM, Kang KK, Kim H, Yoon HJ, Kim JM. The flavone eupatilin inhibits eotaxin expression in an NF-κB-dependent and STAT6-independent manner. Scand J Immunol. 2015; 81:166–176.
Article22. Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999; 11:211–218.
Article23. Wang B, Lim DJ, Han J, Kim YS, Basbaum CB, Li JD. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae upregulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J Biol Chem. 2002; 277:949–957.24. Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, Mengistab A, Dasari V, Hotchkiss J, Harkema J, Basbaum C. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem. 2004; 279:39085–39093.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways
- Phorbol 12-Myristate 13-Acetate Induces MUC16 Expression via PKCdelta and p38 in Human Airway Epithelial Cells
- Interleukin-1beta-Mediated MUC5AC Gene Expression and Mucin Secretion via PKC-ERK/p38-COX-2-PGE2 in Human Airway Epithelial Cells
- Diclofenac Inhibits Phorbol Ester-Induced Gene Expression and Production of MUC5AC Mucin via Affecting Degradation of IkBα and Translocation of NF-kB p65 in NCI-H292 Cells
- Berberine Suppresses Interleukin-1beta-Induced MUC5AC Gene Expression in Human Airway Epithelial Cells