J Breast Cancer.
2019 Dec;22(4):587-598. 10.4048/jbc.2019.22.e47.
Contralateral Breast Cancer and Ipsilateral Breast Tumor Recurrence in BRCA1/2 Carriers and Non-Carriers at High-Risk of Hereditary Breast Cancer
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. ekkim.md@gmail.com
- 2Department of Surgery, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 5Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 6Department of Surgery, Daerim St. Mary's Hospital, Seoul, Korea.
- KMID: 2470895
- DOI: http://doi.org/10.4048/jbc.2019.22.e47
Abstract
- PURPOSE
We evaluated the risk of contralateral breast cancer (CBC) and ipsilateral breast tumor recurrence (IBTR) and investigated the predictive factors for CBC and IBTR in breast cancer patients with BRCA mutations and non-carriers at high-risk of hereditary breast and ovarian cancer (HBOC).
METHODS
We analyzed prospectively collected clinical data of patients with unilateral breast cancer who were at high-risk for HBOC and were tested for the BRCA mutation between 2003 and 2013.
RESULTS
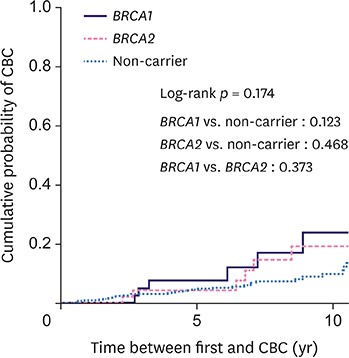
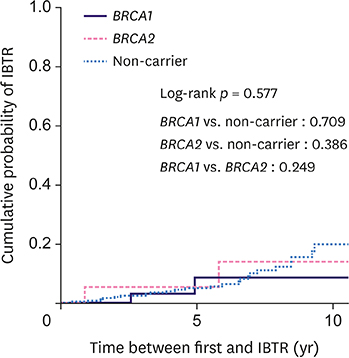
The cohort comprised 540 patients with 45 BRCA1 carriers, 50 BRCA2 carriers, and 445 non-carriers. The median follow-up was 84.5 months. Overall, 61 patients (11.3%) developed CBC (24.4% for BRCA1 carriers, 20% for BRCA2 carriers, and 9% for non-carriers). The 10-year cumulative risk for CBC was 23.8% for BRCA1 carriers, 19.1% for BRCA2 carriers, and 9.8% for non-carriers (p = 0.174). Among the 277 patients who underwent breast-conserving surgery, 29 (10.5%) developed IBTR (9.1% for BRCA1 carriers, 16.7% for BRCA2 carriers, and 10.2% for non-carriers). The 10-year cumulative risk for IBTR for BRCA1 carriers, BRCA2 carriers, and non-carriers was 8.7%, 14.1%, and 20%, respectively (p = 0.577). BRCA1 (hazard ratio [HR], 2.94; 95% confidence interval [CI], 1.20-7.20; p = 0.019) and BRCA2 (HR, 2.88; 95% CI, 1.13-7.35; p = 0.027) mutations and negative estrogen receptor status (HR, 4.02; 95% CI, 1.60-10.08; p = 0.003) were the significant predictive factors for CBC, while tumor size ≥ 2 cm was predictive of IBTR (HR, 6.11; 95% CI, 2.03-18.33; p = 0.001).
CONCLUSION
While BRCA1/2 mutation carriers had a higher risk of developing CBC compared to non-carriers at high-risk of HBOC, the risk of IBTR was similarly high across breast cancer patients irrespective of the BRCA mutation. Further preventive strategies to reduce CBC and IBTR for all patients at high-risk of HBOC should be investigated.
Keyword
MeSH Terms
Figure
Reference
-
1. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996; 77:2318–2324.
Article2. Robson ME, Boyd J, Borgen PI, Cody HS 3rd. Hereditary breast cancer. Curr Probl Surg. 2001; 38:387–480.
Article3. Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003; 72:1117–1130.
Article4. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007; 25:1329–1333.5. Han SA, Park SK, Ahn SH, Son BH, Lee MH, Choi DH, et al. The breast and ovarian cancer risks in Korea due to inherited mutations in BRCA1 and BRCA2: a preliminary report. J Breast Cancer. 2009; 12:92–99.
Article6. Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol. 2010; 28:2404–2410.
Article7. Haffty BG, Harrold E, Khan AJ, Pathare P, Smith TE, Turner BC, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002; 359:1471–1477.
Article8. Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006; 24:2437–2443.
Article9. Turner BC, Harrold E, Matloff E, Smith T, Gumbs AA, Beinfield M, et al. BRCA1/BRCA2 germline mutations in locally recurrent breast cancer patients after lumpectomy and radiation therapy: implications for breast-conserving management in patients with BRCA1/BRCA2 mutations. J Clin Oncol. 1999; 17:3017–3024.
Article10. Garcia-Etienne CA, Barile M, Gentilini OD, Botteri E, Rotmensz N, Sagona A, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: are we approaching an answer? Ann Surg Oncol. 2009; 16:3380–3387.
Article11. Kang E, Kim SW. The Korean hereditary breast cancer study: review and future perspectives. J Breast Cancer. 2013; 16:245–253.
Article12. Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003; 56:1038–1045.
Article13. Metcalfe K, Gershman S, Lynch HT, Ghadirian P, Tung N, Kim-Sing C, et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2011; 104:1384–1392.
Article14. Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, vd Ouweland A, Menke-Pluymers MB, Bartels CC, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007; 43:867–876.
Article15. Reiner AS, John EM, Brooks JD, Lynch CF, Bernstein L, Mellemkjær L, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the women's environmental cancer and radiation epidemiology study. J Clin Oncol. 2013; 31:433–439.
Article16. Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004; 22:2328–2335.17. Graeser MK, Engel C, Rhiem K, Gadzicki D, Bick U, Kast K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009; 27:5887–5892.18. Lizarraga IM, Sugg SL, Weigel RJ, Scott-Conner CE. Review of risk factors for the development of contralateral breast cancer. Am J Surg. 2013; 206:704–708.
Article19. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.
Article20. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232.
Article21. Pierce LJ, Phillips KA, Griffith KA, Buys S, Gaffney DK, Moran MS, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat. 2010; 121:389–398.
Article22. Metcalfe K, Lynch HT, Ghadirian P, Tung N, Kim-Sing C, Olopade OI, et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2011; 127:287–296.
Article23. Kirova YM, Savignoni A, Sigal-Zafrani B, de La Rochefordiere A, Salmon RJ, This P, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat. 2010; 120:119–126.
Article24. Schrag D, Kuntz KM, Garber JE, Weeks JC. Life expectancy gains from cancer prevention strategies for women with breast cancer and BRCA1 or BRCA2 mutations. JAMA. 2000; 283:617–624.
Article25. Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014; 144:443–455.
Article26. Narod SA, Brunet JS, Ghadirian P, Robson M, Heimdal K, Neuhausen SL, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet. 2000; 356:1876–1881.
Article27. Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018; 4:CD002748.
Article28. Eleje GU, Eke AC, Ezebialu IU, Ikechebelu JI, Ugwu EO, Okonkwo OO. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev. 2018; 8:CD012464.
Article29. Yu JH, Lee JW, Son BH, Kim SW, Park SK, Lee MH, et al. Characteristics of BRCA1/2 mutation-positive breast cancers in Korea: a comparison study based on multicenter data and the Korean Breast Cancer Registry. J Breast Cancer. 2014; 17:129–135.
Article30. Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005; 23:7491–7496.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Frequency of BRCA1 and BRCA2 Germline Mutations Detected by Protein Truncation Test and Cumulative Risks of Breast and Ovarian Cancer among Mutation Carriers in Japanese Breast Cancer Families
- BRCA1/BRCA2 Pathogenic Variant Breast Cancer: Treatment and Prevention Strategies
- Trends in Risk-Reducing Mastectomy and Risk-Reducing SalpingoOophorectomy in Korean Carriers of the BRCA1/2 Mutation
- Practice Patterns of Surgeons for the Management of Hereditary Breast Cancer in Korea
- Usage Patterns of Surveillance, Chemoprevention and Risk-Reducing Surgery in Korean BRCA Mutation Carriers: 5 Years of Experience at a Single Institution