J Breast Cancer.
2019 Dec;22(4):522-532. 10.4048/jbc.2019.22.e51.
Kinesin Family Member 11 Enhances the Self-Renewal Ability of Breast Cancer Cells by Participating in the Wnt/β-Catenin Pathway
- Affiliations
-
- 1Shenzhen Long-gang Maternal and Child Health Hospital Centralab, Shenzhen, China.
- 2Department of Pathogen Biology and Immunology, School of Life Sciences and Biopharmaceutics, Guangdong Pharmaceutical University, Guangzhou, China. wlwlan3435@163.com
- KMID: 2470890
- DOI: http://doi.org/10.4048/jbc.2019.22.e51
Abstract
- PURPOSE
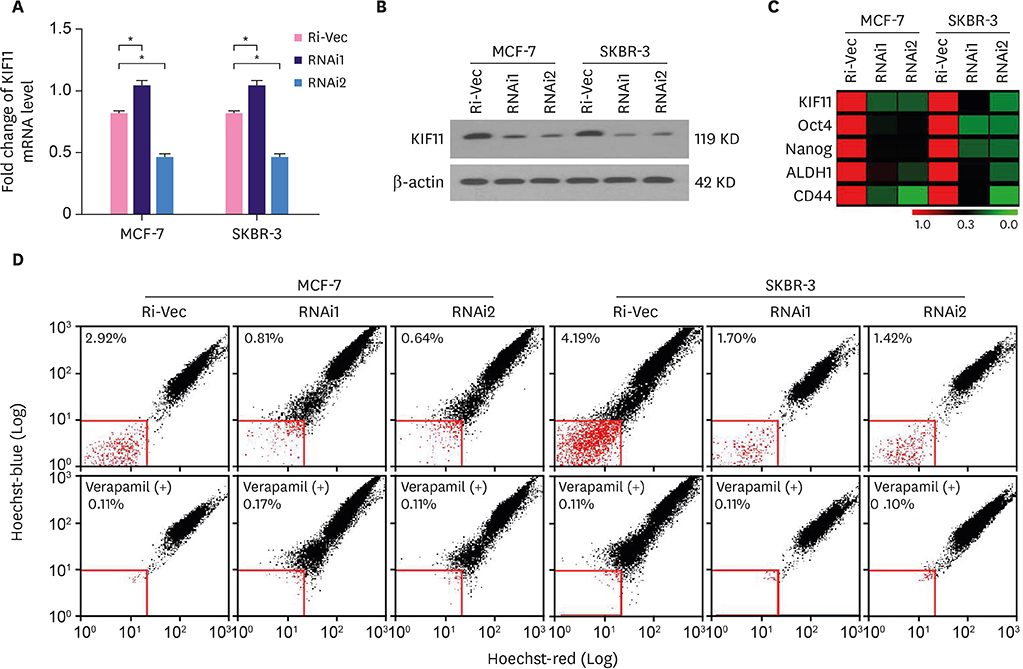
Our previous studies have shown that kinesin family member 11 (KIF11) is markedly overexpressed in human breast cancer cells or tissues and positively correlated with distant metastasis and prognosis in patients with breast cancer, suggesting an important role in the regulation of cancer stem cells. Herein, we examined the role of KIF11 in breast cancer stem cells.
METHODS
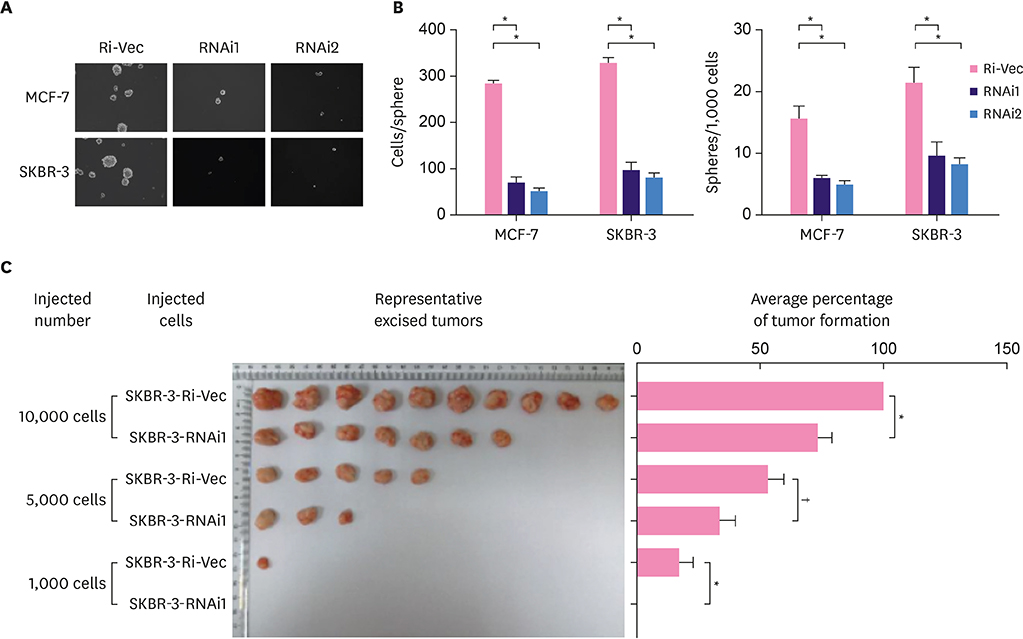
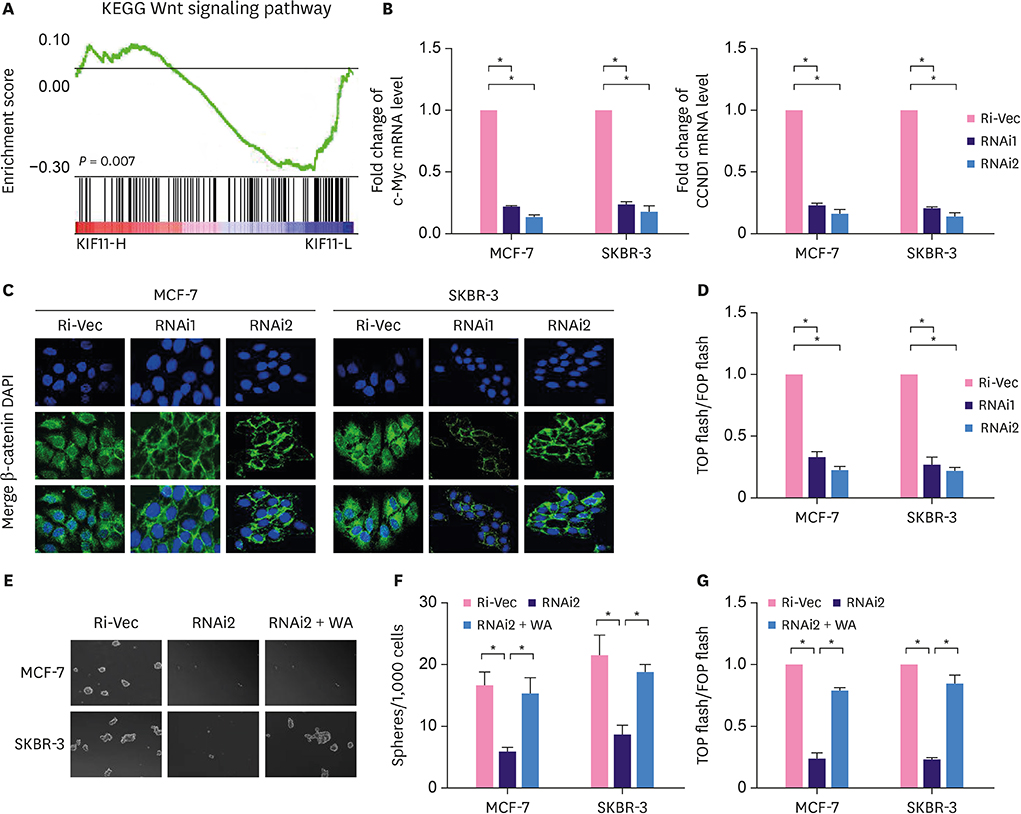
In the current study, we validated our previous findings through analysis of data collected in The Cancer Genome Atlas. Endogenous KIF11 was stably silenced in MCF-7 and SKBR-3 cells. Flow cytometry was used to measure the proportion of side-population (SP) cells. Mammosphere culture and tumor implantation experiments in immunodeficient mice were used to assess the self-renewal ability of breast cancer cells. Real-time polymerase chain reaction, western blot, immunofluorescence staining, luciferase reporter assays and Wnt agonist treatment were conducted to investigate the signaling pathways regulated by KIF11.
RESULTS
We found that the expression level of KIF11 was positively correlated with stem cell-enrichment genes. The proportion of SP cells was significantly reduced in KIF11-silenced cells. Silencing endogenous KIF11 not only reduced the size and number of mammospheres in vitro, but also reduced the ability of breast cancer cells to form tumors in mice. Simultaneously, we found that KIF11 was involved in regulating the activation of the Wnt/β-catenin signaling pathway.
CONCLUSION
Endogenous KIF11 enhances the self-renewal of breast cancer cells by activating the Wnt/β-catenin signaling pathway, thereby enhancing the characteristics of breast cancer stem cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights into breast cancer in the East vs the West: a review. JAMA Oncol. 2019; 5:1489–1496.
Article2. Gonzalez ME, Martin EE, Anwar T, Arellano-Garcia C, Medhora N, Lama A, et al. Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Reports. 2017; 18:1215–1228.
Article3. Zhang GF, Li CX, Liu ZQ, Ma S, Chen HB. Cancer stem cell targets - a review. Eur Rev Med Pharmacol Sci. 2016; 20:2045–2051.4. Tsang JY, Huang YH, Luo MH, Ni YB, Chan SK, Lui PC, et al. Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012; 136:407–417.
Article5. Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012; 14:R15.
Article6. Strati A, Nikolaou M, Georgoulias V, Lianidou ES. Prognostic significance of TWIST1, CD24, CD44, and ALDH1 transcript quantification in EpCAM-positive circulating tumor cells from early stage breast cancer patients. Cells. 2019; 8:E652.7. Wang Y, Zhe H, Ding Z, Gao P, Zhang N, Li G. Cancer stem cell marker Bmi-1 expression is associated with basal-like phenotype and poor survival in breast cancer. World J Surg. 2012; 36:1189–1194.
Article8. Birzele F, Voss E, Nopora A, Honold K, Heil F, Lohmann S, et al. CD44 isoform status predicts response to treatment with anti-CD44 antibody in cancer patients. Clin Cancer Res. 2015; 21:2753–2762.
Article9. Braune EB, Seshire A, Lendahl U. Notch and Wnt dysregulation and its relevance for breast cancer and tumor initiation. Biomedicines. 2018; 6:E101.
Article10. Zardawi SJ, O'Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009; 24:385–398.11. Lai XX, Li G, Lin B, Yang H. Interference of Notch 1 inhibits the proliferation and invasion of breast cancer cells: involvement of the β-catenin signaling pathway. Mol Med Rep. 2018; 17:2472–2478.
Article12. Benvenuto M, Masuelli L, De Smaele E, Fantini M, Mattera R, Cucchi D, et al. In vitro and in vivo inhibition of breast cancer cell growth by targeting the Hedgehog/GLI pathway with SMO (GDC-0449) or GLI (GANT-61) inhibitors. Oncotarget. 2016; 7:9250–9270.
Article13. Pohl SG, Brook N, Agostino M, Arfuso F, Kumar AP, Dharmarajan A. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017; 6:e310.
Article14. Chatterjee S, Sil PC. Targeting the crosstalks of Wnt pathway with Hedgehog and Notch for cancer therapy. Pharmacol Res. 2019; 142:251–261.
Article15. Li G, Su Q, Liu H, Wang D, Zhang W, Lu Z, et al. Frizzled7 promotes epithelial-to-mesenchymal transition and stemness via activating canonical Wnt/β-catenin pathway in gastric cancer. Int J Biol Sci. 2018; 14:280–293.
Article16. Mohammadi-Yeganeh S, Hosseini V, Paryan M. Wnt pathway targeting reduces triple-negative breast cancer aggressiveness through miRNA regulation in vitro and in vivo . J Cell Physiol. 2019; 234:18317–18328.17. Song L, Wang L, Li Y, Xiong H, Wu J, Li J, et al. Sam68 up-regulation correlates with, and its down-regulation inhibits, proliferation and tumourigenicity of breast cancer cells. J Pathol. 2010; 222:227–237.
Article18. Pei YY, Li GC, Ran J, Wei FX. Kinesin family member 11 contributes to the progression and prognosis of human breast cancer. Oncol Lett. 2017; 14:6618–6626.
Article19. Wang L, Tian H, Yuan J, Wu H, Wu J, Zhu X. CONSORT: Sam68 is directly regulated by miR-204 and promotes the self-renewal potential of breast cancer cells by activating the Wnt/beta-catenin signaling pathway. Medicine (Baltimore). 2015; 94:e2228.20. Valensin S, Ghiron C, Lamanna C, Kremer A, Rossi M, Ferruzzi P, et al. KIF11 inhibition for glioblastoma treatment: reason to hope or a struggle with the brain? BMC Cancer. 2009; 9:196.
Article21. Jin Q, Dai Y, Wang Y, Zhang S, Liu G. High kinesin family member 11 expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Clin Pathol. 2019; 72:354–362.
Article22. Imai T, Oue N, Sentani K, Sakamoto N, Uraoka N, Egi H, et al. KIF11 is required for spheroid formation by oesophageal and colorectal cancer cells. Anticancer Res. 2017; 37:47–55.
Article23. Daigo K, Takano A, Thang PM, Yoshitake Y, Shinohara M, Tohnai I, et al. Characterization of KIF11 as a novel prognostic biomarker and therapeutic target for oral cancer. Int J Oncol. 2018; 52:155–165.
Article24. El Fatemi H, Chahbouni S, Jayi S, Moumna K, Melhouf MA, Bannani A, et al. Luminal B tumors are the most frequent molecular subtype in breast cancer of North African women: an immunohistochemical profile study from Morocco. Diagn Pathol. 2012; 7:170.
Article25. Yu XF, Feng WL, Miao LL, Chen B, Yang HJ. The prognostic significance of molecular subtype for male breast cancer: a 10-year retrospective study. Breast. 2013; 22:824–827.
Article26. Katoh M. Canonical and non-canonical Wnt signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (review). Int J Oncol. 2017; 51:1357–1369.
Article27. Tabatabai R, Linhares Y, Bolos D, Mita M, Mita A. Targeting the Wnt pathway in cancer: a review of novel therapeutics. Target Oncol. 2017; 12:623–641.
Article28. Wang YX, Zhang JH, Gu ZW. Wnt/beta-catenin signal pathway and malignant hematological disease -- review. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009; 17:234–237.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circular RNAs Regulate Cancer Onset and Progression via Wnt/β-Catenin Signaling Pathway
- Suppression of β-catenin Signaling Pathway in Human Prostate Cancer PC3 Cells by Delphinidin
- Beta-Catenin Downregulation Contributes to Epidermal Growth Factor-induced Migration and Invasion of MDAMB231 Cells
- Pja2 Inhibits Wnt/β-catenin Signaling by Reducing the Level of TCF/LEF1
- The Effect of (1S,2S,3E,7E,11E)-3,7,11,15-Cembratetraen-17,2-Olide (LS-1) from Lobophyyum sp. on the Apoptosis Induction of SNU-C5 Human Colorectal Cancer Cells