J Breast Cancer.
2020 Feb;23(1):47-58. 10.4048/jbc.2020.23.e11.
Analysis of Tau Protein Expression in Predicting Pathological Complete Response to Neoadjuvant Chemotherapy in Different Molecular Subtypes of Breast Cancer
- Affiliations
-
- 1Department of Breast Cancer, Cancer Center, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China. gzwangkun@126.com
- 2The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China.
- 3Clinical Research Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China.
- 4Scientific Research Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China.
- 5Department of General Surgery, Shenzhen Tradition Chinese Medicine Hospital, Shenzhen, China.
- 6Department of General Surgery, Zhuhai Golden Bay Center Hospital, Zhuhai, China.
- 7Shantou University Medical College, Shantou, China.
- KMID: 2470881
- DOI: http://doi.org/10.4048/jbc.2020.23.e11
Abstract
- PURPOSE
Tau is a microtubule-associated protein that can be found in both normal and abnormal breast cells. Whether the expression of Tau protein can predict the response to neoadjuvant chemotherapy (NACT) is still unclear. In this study, we assessed the role of Tau protein expression in predicting a pathological complete response (pCR) to NACT for different subtypes of breast cancer.
METHODS
Four hundred and sixty-eight eligible patients were retrospectively recruited in our study. The relationship between clinicopathologic factors, including Tau protein expression, and pCR in different subtypes was evaluated using logistic regression analysis. Correlation between Tau and disease-free survival (DFS) and overall survival (OS) was performed using Kaplan-Meier analysis.
RESULTS
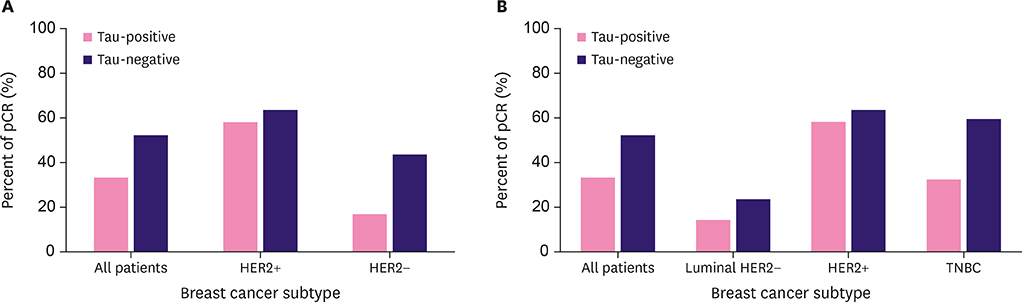
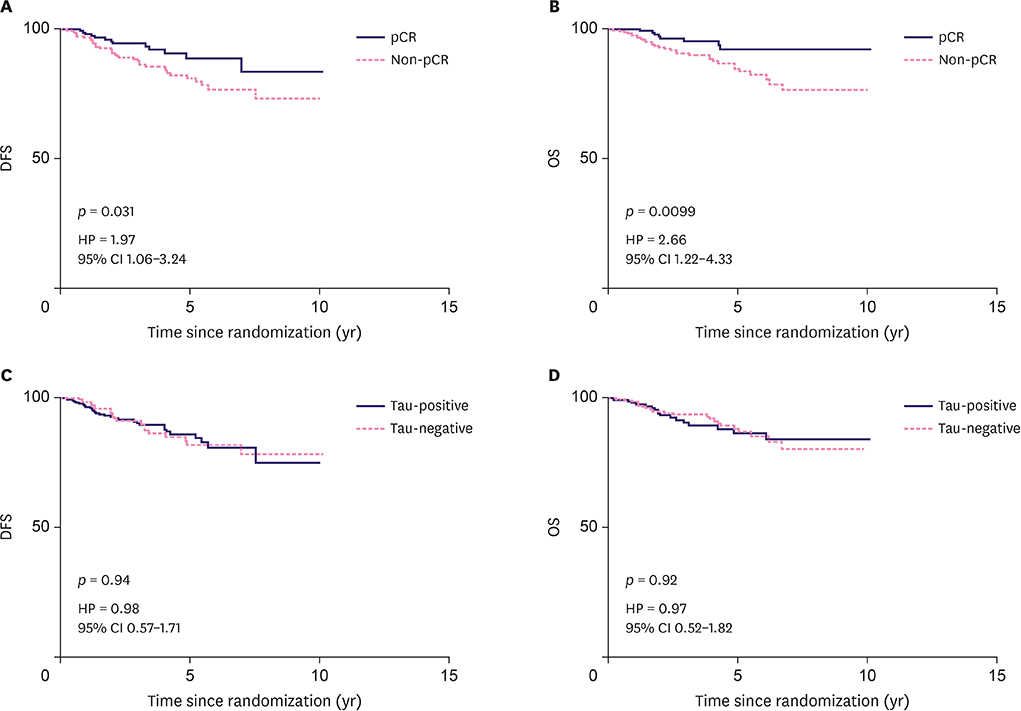
The expression of Tau protein was negatively correlated with pCR, especially in triple-negative breast cancer (TNBC). No significant difference was observed in the luminal human epidermal growth factor receptor-2 (HER2)-negative subtype and HER2-positive subtype. Patients with pCR were associated with better DFS and OS (p < 0.05). However, Tau protein expression had no association with either DFS or OS (p > 0.05).
CONCLUSION
Tau protein expression can predict pCR before NACT in TNBC, but there was no correlation between Tau expression and DFS or OS.
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article2. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018; 16:310–320.
Article3. Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007; 18:1927–1934.
Article4. Andre F, Hatzis C, Anderson K, Sotiriou C, Mazouni C, Mejia J, et al. Microtubule-associated protein-tau is a bifunctional predictor of endocrine sensitivity and chemotherapy resistance in estrogen receptor-positive breast cancer. Clin Cancer Res. 2007; 13:2061–2067.
Article5. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.
Article6. de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014; 15:1137–1146.
Article7. Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016; 17:791–800.
Article8. von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014; 15:747–756.
Article9. McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008; 1785:96–132.
Article10. Russell P, Hennessy BT, Li J, Carey MS, Bast RC, Freeman T, et al. Cyclin G1 regulates the outcome of taxane-induced mitotic checkpoint arrest. Oncogene. 2012; 31:2450–2460.
Article11. Li ZH, Xiong QY, Tu JH, Gong Y, Qiu W, Zhang HQ, et al. Tau proteins expressions in advanced breast cancer and its significance in taxane-containing neoadjuvant chemotherapy. Med Oncol. 2013; 30:591.
Article12. Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005; 102:8315–8320.
Article13. Wang K, Deng QT, Liao N, Zhang GC, Liu YH, Xu FP, et al. Tau expression correlated with breast cancer sensitivity to taxanes-based neoadjuvant chemotherapy. Tumour Biol. 2013; 34:33–38.
Article14. Rody A, Karn T, Gätje R, Ahr A, Solbach C, Kourtis K, et al. Gene expression profiling of breast cancer patients treated with docetaxel, doxorubicin, and cyclophosphamide within the GEPARTRIO trial: HER-2, but not topoisomerase II alpha and microtubule-associated protein tau, is highly predictive of tumor response. Breast. 2007; 16:86–93.
Article15. Kawase M, Toyama T, Takahashi S, Sato S, Yoshimoto N, Endo Y, et al. FOXA1 expression after neoadjuvant chemotherapy is a prognostic marker in estrogen receptor-positive breast cancer. Breast Cancer. 2015; 22:308–316.
Article16. Liu Z, Li Z, Qu J, Zhang R, Zhou X, Li L, et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res. 2019; 25:3538–3547.
Article17. Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, et al. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol. 2009; 27:4287–4292.
Article18. Mazouni C, Peintinger F, Wan-Kau S, Andre F, Gonzalez-Angulo AM, Symmans WF, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007; 25:2650–2655.
Article19. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–4422.
Article20. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017; 67:93–99.
Article21. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018; 19:27–39.22. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
Article23. Pentheroudakis G, Kalogeras KT, Wirtz RM, Grimani I, Zografos G, Gogas H, et al. Gene expression of estrogen receptor, progesterone receptor and microtubule-associated protein Tau in high-risk early breast cancer: a quest for molecular predictors of treatment benefit in the context of a Hellenic Cooperative Oncology Group trial. Breast Cancer Res Treat. 2009; 116:131–143.
Article24. Dumontet C, Krajewska M, Treilleux I, Mackey JR, Martin M, Rupin M, et al. BCIRG 001 molecular analysis: prognostic factors in node-positive breast cancer patients receiving adjuvant chemotherapy. Clin Cancer Res. 2010; 16:3988–3997.
Article25. Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006; 24:4236–4244.
Article26. Peintinger F, Anderson K, Mazouni C, Kuerer HM, Hatzis C, Lin F, et al. Thirty-gene pharmacogenomic test correlates with residual cancer burden after preoperative chemotherapy for breast cancer. Clin Cancer Res. 2007; 13:4078–4082.
Article27. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281.
Article28. Harbeck N, Gluz O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast. 2017; 34:Suppl 1. S99–S103.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Analysis of Tau Protein Expression in Predicting Pathological Complete Response to Neoadjuvant Chemotherapy in Different Molecular Subtypes of Breast Cancer
- Predictive and Prognostic Roles of Pathological Indicators for Patients with Breast Cancer on Neoadjuvant Chemotherapy
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Pathological Response for Resectable Non–Small Cell Lung Cancer Treated with Neoadjuvant Chemotherapy Combined with PD-1 Checkpoint Inhibitors
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€