Korean J Radiol.
2020 Mar;21(3):268-279. 10.3348/kjr.2019.0441.
Cardiac Magnetic Resonance Feature Tracking in Aortic Stenosis: Exploration of Strain Parameters and Prognostic Value in Asymptomatic Patients with Preserved Ejection Fraction
- Affiliations
-
- 1Department of Radiology, Cardiology Division, Seoul National University Hospital, Seoul, Korea. iameuna1@gmail.com
- 2Department of Radiology, SNU-SMG Boramae Medical Center, Seoul, Korea.
- 3Department of Internal Medicine, Cardiology Division, Seoul National University Hospital, Seoul, Korea.
- KMID: 2470750
- DOI: http://doi.org/10.3348/kjr.2019.0441
Abstract
OBJECTIVE
To determine the most valuable cardiac magnetic resonance feature tracking (CMR-FT) parameters for evaluating aortic stenosis (AS) and determine whether they can predict the prognosis in asymptomatic AS patients with preserved ejection fraction (pEF).
MATERIALS AND METHODS
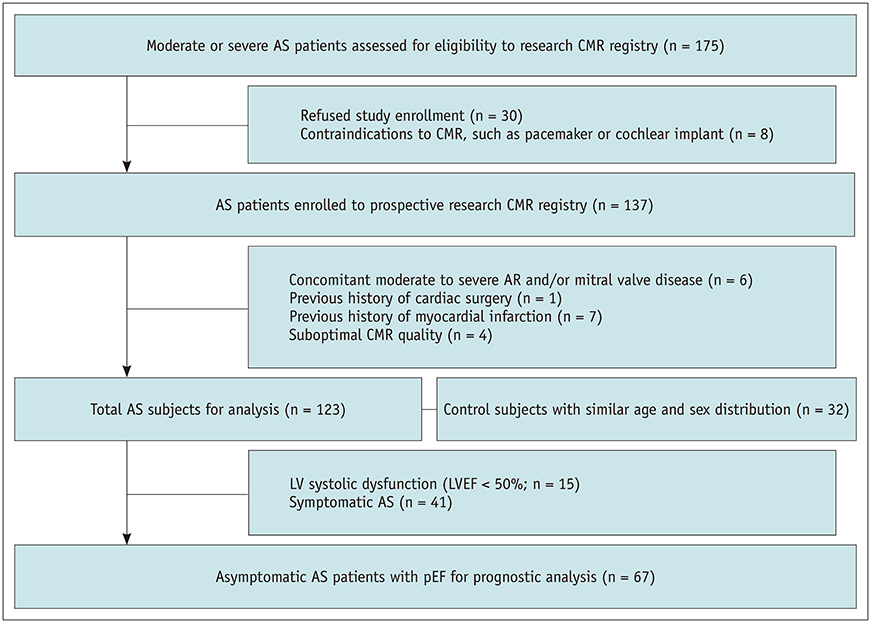
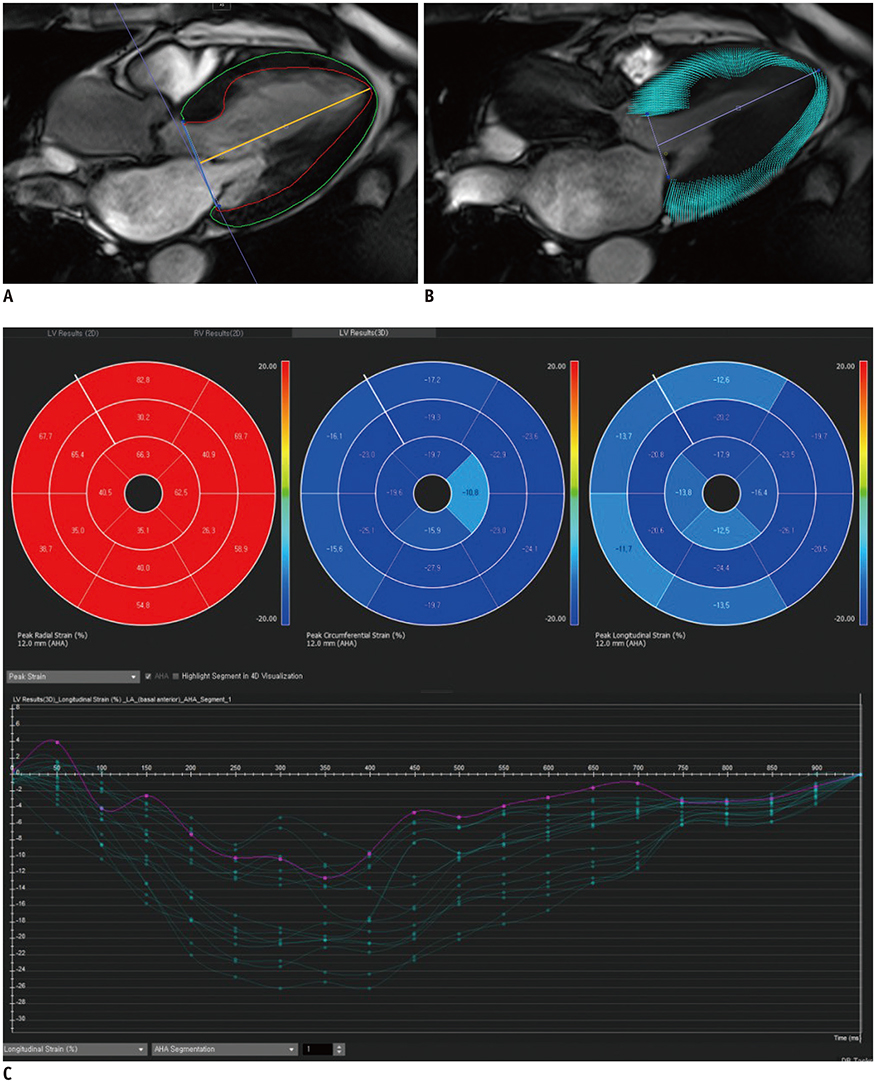
A prospective cohort of 123 moderate to severe AS patients (60 males, 68.6 ± 9.2 years) and 32 control subjects (14 males, 67.9 ± 4.4 years) underwent echocardiography and 3T CMR imaging from 2011-2015. CMR cine images were analyzed using CMR-FT to assess the left ventricular radial, circumferential, and longitudinal peak strain (PS) in 2- and 3-dimensions. The primary endpoints were clinical cardiac events (CCEs), including cardiac death, heart failure, and AS-associated symptom development. For statistical analysis, logistic regression and log-rank tests were used.
RESULTS
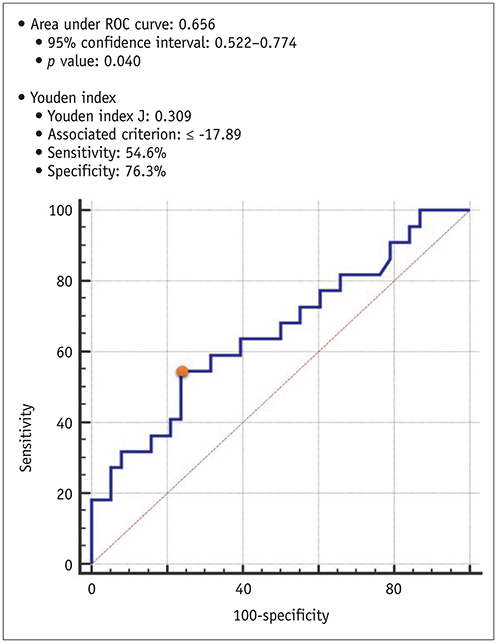
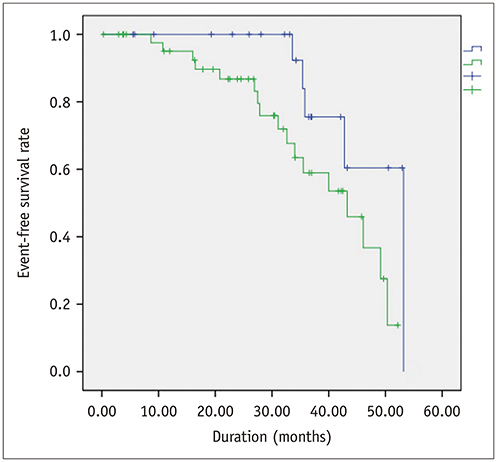
Global PSs differed between AS patients and controls and between severe and moderate AS patients (p < 0.05). Two-dimensional (2D) global radial and longitudinal PSs changed gradually with the severity of AS groups (p < 0.001). Twenty-two of 67 asymptomatic AS patients with pEF experienced CCEs during the follow-up (median: 31.1 months). 2D global longitudinal PS (GLPS) was the single risk factor for CCE (p = 0.017). The relative risk for CCE was 3.9 (p = 0.016, 95% confidence interval: 1.2-11.9) based on 2D GLPS with a cutoff of −17.9% according to receiver operating characteristic curve analysis. Survival analysis demonstrated that asymptomatic AS patients with pEF having impaired 2D GLPS experienced worse event-free survival than the others (p = 0.041).
CONCLUSION
2D global longitudinal and radial PSs may reflect cardiac dysfunction according to the degree of AS. 2D GLPS might be a prognostic predictor of CCEs in asymptomatic AS patients with pEF.
MeSH Terms
Figure
Reference
-
1. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000; 343:611–617.
Article2. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016; 37:1196–1207.
Article3. Padiyath A, Gribben P, Abraham JR, Li L, Rangamani S, Schuster A, et al. Echocardiography and cardiac magnetic resonance-based feature tracking in the assessment of myocardial mechanics in tetralogy of Fallot: an intermodality comparison. Echocardiography. 2013; 30:203–210.
Article4. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010; 3:144–151.
Article5. Schuster A, Kutty S, Padiyath A, Parish V, Gribben P, Danford DA, et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson. 2011; 13:58.
Article6. Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, et al. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol. 2013; 166:413–420.
Article7. Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015; 16:307–315.
Article8. Lee H, Park JB, Yoon YE, Park EA, Kim HK, Lee W, et al. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging. 2018; 11:974–983.
Article9. Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006; 114:e84–e231.10. Seo HY, Lee SP, Park JB, Lee JM, Park EA, Chang SA, et al. Discrepancies in left ventricular mass calculation based on echocardiography and cardiovascular magnetic resonance measurements in patients with left ventricular hypertrophy. J Am Soc Echocardiogr. 2015; 28:1194–1203.e2.
Article11. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018; 137:961–972.
Article12. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). Circulation. 2015; 132:302–361.13. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008; 10:35.
Article14. Schuster A, Stahnke VC, Unterberg-Buchwald C, Kowallick JT, Lamata P, Steinmetz M, et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol. 2015; 70:989–998.
Article15. Hair JF Jr, Anderson RE, Tatham RL, Black WC. Multivariate data analysis. 5th ed. Upper Saddle River, NJ: Prentice Hall;1998.16. Miyazaki S, Daimon M, Miyazaki T, Onishi Y, Koiso Y, Nishizaki Y, et al. Global longitudinal strain in relation to the severity of aortic stenosis: a two-dimensional speckle-tracking study. Echocardiography. 2011; 28:703–708.
Article17. Al Musa T, Uddin A, Swoboda PP, Garg P, Fairbairn TA, Dobson LE, et al. Myocardial strain and symptom severity in severe aortic stenosis: insights from cardiovascular magnetic resonance. Quant Imaging Med Surg. 2017; 7:38–47.18. Carstensen HG, Larsen LH, Hassager C, Kofoed KF, Dalsgaard M, Kristensen CB, et al. Tissue velocities and myocardial deformation in asymptomatic and symptomatic aortic stenosis. J Am Soc Echocardiogr. 2015; 28:969–980.19. Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging. 2015; 8:1444–1460.20. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. Prognostic impact of left ventricular ejection fraction in patients with severe aortic stenosis. JACC Cardiovasc Interv. 2018; 11:145–157.21. Minamino-Muta E, Kato T, Morimoto T, Taniguchi T, Inoko M, Haruna T, et al. Impact of the left ventricular mass index on the outcomes of severe aortic stenosis. Heart. 2017; 103:1992–1999.
Article22. Casaclang-Verzosa G, Malouf JF, Scott CG, Juracan EM, Nishimura RA, Pellikka PA. Does left atrial size predict mortality in asymptomatic patients with severe aortic stenosis? Echocardiography. 2010; 27:105–109.
Article23. Lee SP, Lee W, Lee JM, Park EA, Kim HK, Kim YJ, et al. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology. 2015; 274:359–369.
Article24. Hwang JW, Kim SM, Park SJ, Cho EJ, Kim EK, Chang SA, et al. Assessment of reverse remodeling predicted by myocardial deformation on tissue tracking in patients with severe aortic stenosis: a cardiovascular magnetic resonance imaging study. J Cardiovasc Magn Reson. 2017; 19:80.
Article25. Buckert D, Cieslik M, Tibi R, Radermacher M, Rasche V, Bernhardt P, et al. Longitudinal strain assessed by cardiac magnetic resonance correlates to hemodynamic findings in patients with severe aortic stenosis and predicts positive remodeling after transcatheter aortic valve replacement. Clin Res Cardiol. 2018; 107:20–29.
Article26. Blanken CPS, Farag ES, Boekholdt SM, Leiner T, Kluin J, Nederveen AJ, et al. Advanced cardiac MRI techniques for evaluation of left-sided valvular heart disease. J Magn Reson Imaging. 2018; 48:318–329.
Article27. Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009; 120:577–584.
Article28. Leitman M, Lysiansky M, Lysyansky P, Friedman Z, Tyomkin V, Fuchs T, et al. Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr. 2010; 23:64–70.
Article29. Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, et al. Two-dimensional strain--a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005; 18:1247–1253.
Article30. Götte MJ, Germans T, Rüssel IK, Zwanenburg JJ, Marcus JT, van Rossum AC, et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol. 2006; 48:2002–2011.31. Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009; 11:55.
Article32. Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002; 106:50–56.33. Taylor RJ, Moody WE, Umar F, Edwards NC, Taylor TJ, Stegemann B, et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging. 2015; 16:871–881.
Article34. Markl M, Reeder SB, Chan FP, Alley MT, Herfkens RJ, Pelc NJ. Steady-state free precession MR imaging: improved myocardial tag persistence and signal-to-noise ratio for analysis of myocardial motion. Radiology. 2004; 230:852–861.
Article35. Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016; 9:e004077.
Article36. Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016; 18:51.
Article37. Singh A, Steadman CD, Khan JN, Horsfield MA, Bekele S, Nazir SA, et al. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging. 2015; 41:1129–1137.
Article38. Wu L, Germans T, Güçlü A, Heymans MW, Allaart CP, van Rossum AC. Feature tracking compared with tissue tagging measurements of segmental strain by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2014; 16:10.
Article39. Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol. 2015; 84:840–848.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Principles and Clinical Applications of Feature-Tracking Cardiac Magnetic Resonance Imaging: A Literature Review
- Exercise Echocardiography in Asymptomatic Patients with Severe Aortic Stenosis and Preserved Left Ventricular Ejection Fraction
- Arterial-Cardiac Interaction: The Concept and Implications
- Cardiac Strain Analysis Using Cine Magnetic Resonance Imaging and Computed Tomography
- Magnetic Resonance Myocardial Feature Tracking in TransfusionDependent Myelodysplastic Syndrome