J Korean Med Sci.
2016 Nov;31(11):1711-1716. 10.3346/jkms.2016.31.11.1711.
Risk Factors for the Adverse Events after Conversion from Twice-Daily to Once-Daily Tacrolimus in Stable Liver Transplantation Patients
- Affiliations
-
- 1Department of Surgery, College of Medicine, Seoul National University, Seoul, Korea. Kwleegs@gmail.com
- 2Department of Surgery, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 2470265
- DOI: http://doi.org/10.3346/jkms.2016.31.11.1711
Abstract
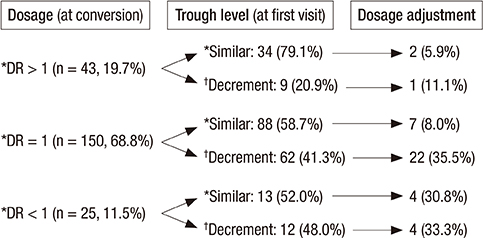
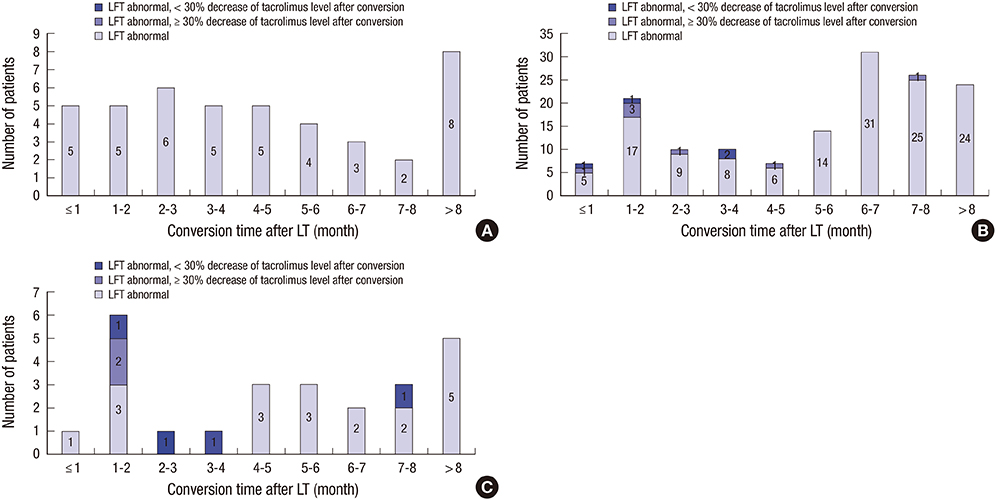
- Despite the therapeutic equivalence between twice-daily and once-daily tacrolimus, patient safety after conversion is still a concern. We reviewed 218 liver transplantation (LT) patients who converted twice-daily to once-daily tacrolimus between May 2011 and January 2014. Thirty (13.8%) patients had adverse events after conversion, with a liver function test (LFT) abnormality being the most common adverse event (n = 17). Despite the decrease in serum tacrolimus of > 30% after conversion, none of the patients who were converted to a dosage ratio (once-daily tacrolimus dosage: twice-daily tacrolimus dosage) > 1 had an LFT abnormality. Most patients with an LFT abnormality improved after increasing the once-daily tacrolimus dosage (n = 2), returned to a previous medication, and/or added another immunosuppressant (n = 15). One patient had acute cellular rejection, which improved after steroid pulse treatment, and another patient had graft failure. In patients with a dosage ratio ≤ 1, the conversion time within 5 years after LT was the only significant risk factor for an LFT abnormality after conversion (odds ratio: 11.850, 95% confidence interval: 1.321-106.325, P = 0.027). In conclusion, the dosage ratio and time after LT should be carefully considered during conversion from twice-daily to once-daily tacrolimus.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Child
Drug Administration Schedule
Female
Graft Rejection/*prevention & control
Humans
Immunosuppressive Agents/blood/*therapeutic use
Liver Failure/therapy
Liver Function Tests
Liver Transplantation/*adverse effects
Male
Middle Aged
Multivariate Analysis
Odds Ratio
Retrospective Studies
Risk Factors
Tacrolimus/blood/*therapeutic use
Young Adult
Figure
Cited by 1 articles
-
Efficacy and safety of a switch from twice-daily tacrolimus to once-daily generic tacrolimus in stable liver transplant patients
Jong Man Kim, Pyoung-Jae Park, Geun Hong, Dong Jin Joo, Kwan Woo Kim, Je Ho Ryu, Young Seok Han, Jai Young Cho, Gi-Won Song, Bong-Wan Kim, Dong-Sik Kim, Seong Hoon Kim, Sang Tae Choi, Young Kyoung You, Kyung-Suk Suh, Yang-Won Na, Koo Jeong Kang, Jae-Won Joh
Korean J Transplant. 2021;35(3):168-176. doi: 10.4285/kjt.21.0012.
Reference
-
1. Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004; 77:769–776.2. O’Carroll RE, McGregor LM, Swanson V, Masterton G, Hayes PC. Adherence to medication after liver transplantation in Scotland: a pilot study. Liver Transpl. 2006; 12:1862–1868.3. Comuzzi C, Lorenzin D, Rossetto A, Faraci MG, Nicolini D, Garelli P, Bresadola V, Toniutto P, Soardo G, Baroni GS, et al. Safety of conversion from twice-daily tacrolimus (Prograf) to once-daily prolonged-release tacrolimus (Advagraf) in stable liver transplant recipients. Transplant Proc. 2010; 42:1320–1321.4. Mor E, Gonwa TA, Husberg BS, Goldstein RM, Klintmalm GB. Late-onset acute rejection in orthotopic liver transplantation--associated risk factors and outcome. Transplantation. 1992; 54:821–824.5. Burra P, Germani G, Gnoato F, Lazzaro S, Russo FP, Cillo U, Senzolo M. Adherence in liver transplant recipients. Liver Transpl. 2011; 17:760–770.6. Trunečka P, Boillot O, Seehofer D, Pinna AD, Fischer L, Ericzon BG, Troisi RI, Baccarani U, Ortiz de Urbina J, Wall W, et al. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010; 10:2313–2323.7. Morrissey PE, Flynn ML, Lin S. Medication noncompliance and its implications in transplant recipients. Drugs. 2007; 67:1463–1481.8. Heffron TG, Pescovitz MD, Florman S, Kalayoglu M, Emre S, Smallwood G, Wisemandle K, Anania C, Dhadda S, Sawamoto T, et al. Once-daily tacrolimus extended-release formulation: 1-year post-conversion in stable pediatric liver transplant recipients. Am J Transplant. 2007; 7:1609–1615.9. Kim H, Yi NJ, Lee J, Kim J, Moon MR, Jeong J, Lee JM, You TS, Suh SW, Park MS, et al. Safety of reduced dose of mycophenolate mofetil combined with tacrolimus in living-donor liver transplantation. Clin Mol Hepatol. 2014; 20:291–299.10. Giannelli V, Rossi M, Giusto M, Lucidi C, Lattanzi B, Ruffa A, Ginanni Corradini S, Mennini G, Melandro F, Lai Q, et al. Conversion from twice-daily to once-daily Tacrolimus administration in liver transplant patient: results of long term follow-up. Eur Rev Med Pharmacol Sci. 2013; 17:2718–2720.11. Florman S, Alloway R, Kalayoglu M, Lake K, Bak T, Klein A, Klintmalm G, Busque S, Brandenhagen D, Lake J, et al. Conversion of stable liver transplant recipients from a twice-daily Prograf-based regimen to a once-daily modified release tacrolimus-based regimen. Transplant Proc. 2005; 37:1211–1213.12. Beckebaum S, Iacob S, Sweid D, Sotiropoulos GC, Saner F, Kaiser G, Radtke A, Klein CG, Erim Y, de Geest S, et al. Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 2011; 24:666–675.13. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001; 23:1296–1310.14. Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, Dobbels F, Vanrenterghem Y, Kanaan N; ADMIRAD Study Team. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013; 95:333–340.15. Doesch AO, Mueller S, Akyol C, Erbel C, Frankenstein L, Ruhparwar A, Ehlermann P, Dengler TJ, Katus HA. Increased adherence eight months after switch from twice daily calcineurin inhibitor based treatment to once daily modified released tacrolimus in heart transplantation. Drug Des Devel Ther. 2013; 7:1253–1258.16. Fischer L, Trunečka P, Gridelli B, Roy A, Vitale A, Valdivieso A, Varo E, Seehofer D, Lynch S, Samuel D, et al. Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: a randomized, open-label trial. Liver Transpl. 2011; 17:167–177.17. Tinti F, Meçule A, Poli L, Bachetoni A, Umbro I, Brunini F, Barile M, Nofroni I, Berloco PB, Mitterhofer AP. Improvement of graft function after conversion to once daily tacrolimus of stable kidney transplant patients. Transplant Proc. 2010; 42:4047–4048.18. Sańko-Resmer J, Boillot O, Wolf P, Thorburn D. Renal function, efficacy and safety postconversion from twice- to once-daily tacrolimus in stable liver recipients: an open-label multicenter study. Transpl Int. 2012; 25:283–293.19. Zhang YF, Chen XY, Dai XJ, Leng XS, Zhong DF. Pharmacokinetics of tacrolimus converted from twice-daily formulation to once-daily formulation in Chinese stable liver transplant recipients. Acta Pharmacol Sin. 2011; 32:1419–1423.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modified Release Tacrolimus
- Risk of graft loss on once-daily versus twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- Single Center Experiences of Conversion from Twice-daily Tacrolimus (Prograf) to Once-daily Tacrolimus (Advagraf) in Stable Liver Transplant Recipients
- Efficacy and safety of a switch from twicedaily tacrolimus to once-daily generic tacrolimus in stable liver transplant patients
- Evaluation of the efficacy and safety of conversion from the tacrolimus capsule to tablet in stable liver transplant recipients with maintenance therapy: a 24-week, open-label, single-center, phase IV exploratory clinical study