J Korean Med Sci.
2016 Sep;31(9):1403-1412. 10.3346/jkms.2016.31.9.1403.
Renoprotective Effects of a Highly Selective A3 Adenosine Receptor Antagonist in a Mouse Model of Adriamycin-induced Nephropathy
- Affiliations
-
- 1Department of Nephrology, Korea University Ansan Hospital, Ansan, Korea. starch70@korea.ac.kr
- 2Department of Nephrology, Wonkwang University Sanbon Hospital, Gunpo, Korea.
- 3Department of Pathology, Inha University Medical College, Incheon, Korea.
- 4Department of Pharmacy, College of Pharmacy, Seoul National University, Seoul, Korea.
- KMID: 2468272
- DOI: http://doi.org/10.3346/jkms.2016.31.9.1403
Abstract
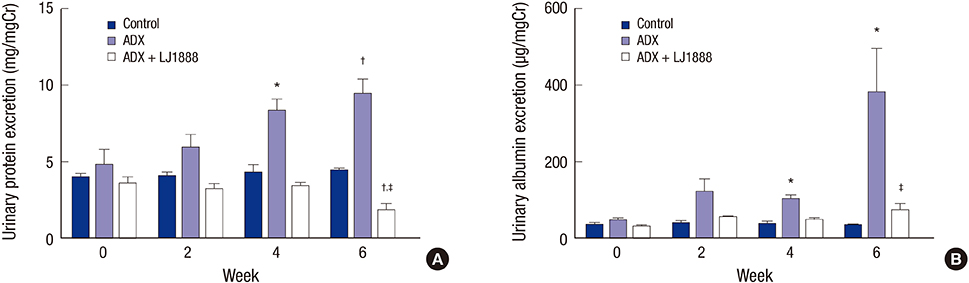
- The concentration of adenosine in the normal kidney increases markedly during renal hypoxia, ischemia, and inflammation. A recent study reported that an A3 adenosine receptor (A3AR) antagonist attenuated the progression of renal fibrosis. The adriamycin (ADX)-induced nephropathy model induces podocyte injury, which results in severe proteinuria and progressive glomerulosclerosis. In this study, we investigated the preventive effect of a highly selective A3AR antagonist (LJ1888) in ADX-induced nephropathy. Three groups of six-week-old Balb/c mice were treated with ADX (11 mg/kg) for four weeks and LJ1888 (10 mg/kg) for two weeks as following: 1) control; 2) ADX; and 3) ADX + LJ1888. ADX treatment decreased body weight without a change in water and food intake, but this was ameliorated by LJ1888 treatment. Interestingly, LJ1888 lowered plasma creatinine level, proteinuria, and albuminuria, which had increased during ADX treatment. Furthermore, LJ1888 inhibited urinary nephrin excretion as a podocyte injury marker, and urine 8-isoprostane and kidney lipid peroxide concentration, which are markers of oxidative stress, increased after injection of ADX. ADX also induced the activation of proinflammatory and profibrotic molecules such as TGF-β1, MCP-1, PAI-1, type IV collagen, NF-κB, NOX4, TLR4, TNFα, IL-1β, and IFN-γ, but they were remarkably suppressed after LJ1888 treatment. In conclusion, our results suggest that LJ1888 has a renoprotective effect in ADX-induced nephropathy, which might be associated with podocyte injury through oxidative stress. Therefore, LJ1888, a selective A3AR antagonist, could be considered as a potential therapeutic agent in renal glomerular diseases which include podocyte injury and proteinuria.
MeSH Terms
-
Actins/metabolism
Adenosine/pharmacology/*therapeutic use
Adenosine A3 Receptor Antagonists/pharmacology/*therapeutic use
Albuminuria/prevention & control
Animals
Body Weight/drug effects
Creatinine/blood
Dinoprost/analogs & derivatives/urine
Disease Models, Animal
Doxorubicin/toxicity
Immunohistochemistry
Kidney/pathology
Kidney Diseases/chemically induced/*drug therapy
Lipid Peroxidation/drug effects
Male
Membrane Proteins/urine
Mice
Mice, Inbred BALB C
NF-kappa B/genetics/metabolism
Oxidative Stress/drug effects
Plasminogen Activator Inhibitor 1/genetics/metabolism
Proteinuria/prevention & control
Transforming Growth Factor beta1/genetics/metabolism
Actins
Adenosine A3 Receptor Antagonists
Membrane Proteins
NF-kappa B
Plasminogen Activator Inhibitor 1
Doxorubicin
Creatinine
Dinoprost
Adenosine
Figure
Reference
-
1. Vallon V, Mühlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006; 86:901–940.2. Hirschhorn R, Roegner-Maniscalco V, Kuritsky L, Rosen FS. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981; 68:1387–1393.3. Cronstein BN, Naime D, Firestein G. The antiinflammatory effects of an adenosine kinase inhibitor are mediated by adenosine. Arthritis Rheum. 1995; 38:1040–1045.4. Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006; 5:247–264.5. Brandon CI, Vandenplas M, Dookwah H, Murray TF. Cloning and pharmacological characterization of the equine adenosine A3 receptor. J Vet Pharmacol Ther. 2006; 29:255–263.6. Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S. The A3 adenosine receptor: history and perspectives. Pharmacol Rev. 2015; 67:74–102.7. Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol Renal Physiol. 2000; 278:F380–7.8. Lee HT, Ota-Setlik A, Xu H, D’Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003; 284:F267–73.9. Lee J, Hwang I, Lee JH, Lee HW, Jeong LS, Ha H. The selective A3AR antagonist LJ-1888 ameliorates UUO-induced tubulointerstitial fibrosis. Am J Pathol. 2013; 183:1488–1497.10. Jeong LS, Choe SA, Gunaga P, Kim HO, Lee HW, Lee SK, Tosh DK, Patel A, Palaniappan KK, Gao ZG, et al. Discovery of a new nucleoside template for human A3 adenosine receptor ligands: D-4′-thioadenosine derivatives without 4′-hydroxymethyl group as highly potent and selective antagonists. J Med Chem. 2007; 50:3159–3162.11. Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, Kim HK, Han JY, Cha DR. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008; 74:890–900.12. Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000; 58:1797–1804.13. Szalay CI, Erdélyi K, Kökény G, Lajtár E, Godó M, Révész C, Kaucsár T, Kiss N, Sárközy M, Csont T, et al. Oxidative/nitrative stress and inflammation drive progression of doxorubicin-induced renal fibrosis in rats as revealed by comparing a normal and a fibrosis-resistant rat strain. PLoS One. 2015; 10:e0127090.14. Deman A, Ceyssens B, Pauwels M, Zhang J, Houte KV, Verbeelen D, Van den Branden C. Altered antioxidant defence in a mouse adriamycin model of glomerulosclerosis. Nephrol Dial Transplant. 2001; 16:147–150.15. Montuschi P, Barnes PJ, Roberts LJ 2nd. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004; 18:1791–1800.16. Machado JR, Rocha LP, Neves PD, Cobô Ede C, Silva MV, Castellano LR, Corrêa RR, Reis MA. An overview of molecular mechanism of nephrotic syndrome. Int J Nephrol. 2012; 2012:937623.17. Jeansson M, Björck K, Tenstad O, Haraldsson B. Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol. 2009; 20:114–122.18. Fujimura T, Yamagishi S, Ueda S, Fukami K, Shibata R, Matsumoto Y, Kaida Y, Hayashida A, Koike K, Matsui T, et al. Administration of pigment epithelium-derived factor (PEDF) reduces proteinuria by suppressing decreased nephrin and increased VEGF expression in the glomeruli of adriamycin-injected rats. Nephrol Dial Transplant. 2009; 24:1397–1406.19. Lee VW, Harris DC. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology (Carlton). 2011; 16:30–38.20. Vallon V, Osswald H. Adenosine receptors and the kidney. In : Wilson CN, Mustafa SJ, editors. Adenosine Receptors in Health and Disease. Dordrecht: Springer;2009. p. 443–470.21. Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011; 22:890–901.22. Tang J, Jiang X, Zhou Y, Xia B, Dai Y. Increased adenosine levels contribute to ischemic kidney fibrosis in the unilateral ureteral obstruction model. Exp Ther Med. 2015; 9:737–743.23. Leshem-Lev D, Hochhauser E, Chanyshev B, Isak A, Shainberg A. Adenosine A(1) and A (3) receptor agonists reduce hypoxic injury through the involvement of P38 MAPK. Mol Cell Biochem. 2010; 345:153–160.24. Varani K, Vincenzi F, Tosi A, Targa M, Masieri FF, Ongaro A, De Mattei M, Massari L, Borea PA. Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes. Br J Pharmacol. 2010; 160:101–115.25. Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Baraldi PG, Borea PA. Modulation of the Akt/Ras/Raf/MEK/ERK pathway by A3 adenosine receptor. Purinergic Signal. 2006; 2:627–632.26. Doné SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K. Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int. 2008; 73:697–704.27. Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003; 23:4917–4928.28. Ma FY, Flanc RS, Tesch GH, Han Y, Atkins RC, Bennett BL, Friedman GC, Fan JH, Nikolic-Paterson DJ. A pathogenic role for c-Jun amino-terminal kinase signaling in renal fibrosis and tubular cell apoptosis. J Am Soc Nephrol. 2007; 18:472–484.29. Pat B, Yang T, Kong C, Watters D, Johnson DW, Gobe G. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney Int. 2005; 67:931–943.30. Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ. The role of p38α mitogen-activated protein kinase activation in renal fibrosis. J Am Soc Nephrol. 2004; 15:370–379.31. Min HS, Kim JE, Lee MH, Song HK, Lee MJ, Lee JE, Kim HW, Cha JJ, Hyun YY, Han JY, et al. Effects of Toll-like receptor antagonist 4,5-dihydro-3-phenyl-5-isoxasole acetic acid on the progression of kidney disease in mice on a high-fat diet. Kidney Res Clin Pract. 2014; 33:33–44.32. Mkaddem SB, Bens M, Vandewalle A. Differential activation of toll-like receptor-mediated apoptosis induced by hypoxia. Oncotarget. 2010; 1:741–750.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LJ-2698, an Adenosine A3 Receptor Antagonist, Alleviates Elastase-Induced Pulmonary Emphysema in Mice
- Protective Roles of Adenosine A1 Receptor Agonist and KATP Channel Opener in Adriamycin induced Cardiac Toxicity
- Antinociceptive Effects of Intrathecal Adenosine Receptors Subtype Agonists in the Formalin Test
- The Role of the Adenosine Receptor Subtypes and Protein Kinase C in Ischemic Preconditioning in the in Vivo Cat Heart
- Effect of Antioxidant and NMDA Receptor Antagonist on Mercury-Induced Toxicity in Cultured Preimplantation Embryos