J Korean Med Sci.
2016 Oct;31(10):1571-1578. 10.3346/jkms.2016.31.10.1571.
Prognostic Impact of Changes in Adipose Tissue Areas after Colectomy in Colorectal Cancer Patients
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. kjparkmd@plaza.snu.ac.kr
- 2Department of Surgery, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea.
- 3Office of Medical Education, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2468247
- DOI: http://doi.org/10.3346/jkms.2016.31.10.1571
Abstract
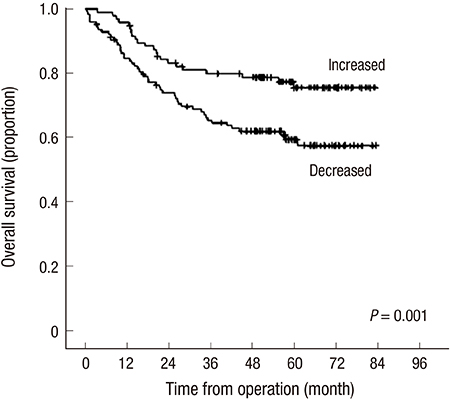
- There have been few studies assessing the changes in the body components of patients after colectomy in colorectal cancer (CRC). The purpose of this study was to verify the trends in the adipose tissue areas of CRC patients before and after surgery and to determine their clinical relevance. Computed tomography (CT)-assessed subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) areas were recorded before and after curative resection in stage I to III CRC patients. Changes in the adipose tissue were assessed by calculating the difference in the adipose tissue area between preoperative CT and the most recent postoperative CT, which is disease-free state. Regarding obesity before surgery, there were no prognostic effect of body mass index (BMI), VAT and SAT, and 47.3% of patients had increases in VAT after colectomy. By multivariate analysis, adjusting sex, age, stage, differentiation, VAT change was the only obesity related factor to predict the prognosis, that patients who had increase in VAT after colectomy had better overall survival (HR, 0.557; 95% CI, 0.317-0.880) and disease-free survival (HR, 0.602; 95% CI, 0.391-0.927). BMI and SAT change had no significant association. In subgroup analysis of stage III CRC patients, VAT change had significance for prognosis only in patients who had adjuvant chemotherapy but not in those who did not receive postoperative chemotherapy. Increase in visceral adipose tissue after surgery is a favorable predictor of prognosis for CRC patients.
Keyword
MeSH Terms
-
Aged
Antineoplastic Agents/therapeutic use
Body Mass Index
Colectomy
Colorectal Neoplasms/drug therapy/mortality/pathology/*surgery
Disease-Free Survival
Female
Follow-Up Studies
Humans
Intra-Abdominal Fat/*diagnostic imaging
Kaplan-Meier Estimate
Male
Middle Aged
Multivariate Analysis
Neoplasm Staging
Obesity/etiology
Prognosis
Proportional Hazards Models
Risk Factors
Subcutaneous Fat/*diagnostic imaging
Tomography, X-Ray Computed
Antineoplastic Agents
Figure
Reference
-
1. Fuzun M, Terzi C, Sokmen S, Unek T, Haciyanli M. Potentially curative resection for locoregional recurrence of colorectal cancer. Surg Today. 2004; 34:907–912.2. Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ, Wolmark N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006; 98:1647–1654.3. Doria-Rose VP, Newcomb PA, Morimoto LM, Hampton JM, Trentham-Dietz A. Body mass index and the risk of death following the diagnosis of colorectal cancer in postmenopausal women (United States). Cancer Causes Control. 2006; 17:63–70.4. Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010; 16:1884–1893.5. Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB 3rd, Macdonald JS, Fuchs CS. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003; 98:484–495.6. Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008; 26:4109–4115.7. Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, McCollum AD, Brady D, O'Connell MJ, Mayer RJ, Cummings B, Willett C, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004; 22:648–657.8. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med. 1980; 69:491–497.9. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer;2002.10. Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D; National Cancer Institute Expert Panel. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001; 93:583–596.11. Yarbro JW, Page DL, Fielding LP, Partridge EE, Murphy GP. American Joint Committee on Cancer prognostic factors consensus conference. Cancer. 1999; 86:2436–2446.12. Chung SJ, Kim D, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008; 57:1360–1365.13. Tanaka S, Togashi K, Rankinen T, Pérusse L, Leon AS, Rao DC, Skinner JS, Wilmore JH, Després JP, Bouchard C. Sex differences in the relationships of abdominal fat to cardiovascular disease risk among normal-weight white subjects. Int J Obes Relat Metab Disord. 2004; 8:320–323.14. Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes. 2008; 32:619–628.15. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998; 34:503–509.16. Innominato PF, Giacchetti S, Moreau T, Bjarnason GA, Smaaland R, Focan C, Garufi C, Iacobelli S, Tampellini M, Tumolo S, et al. Fatigue and weight loss predict survival on circadian chemotherapy for metastatic colorectal cancer. Cancer. 2013; 119:2564–2573.17. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000; 21:697–738.18. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013; 62:933–947.19. Douglass HO Jr, Lavin PT, Woll J, Conroy JF, Carbone P. Chemotherapy of advanced measurable colon and rectal carcinoma with oral 5-fluorouracil, alone or in combination with cyclophosphamide or 6-thioguanine, with intravenous 5-fluorouracil or beta-2′-deoxythioguanosine or with oral 3(4-methyl-cyclohexyl)-1(2-chlorethyl)-1-nitrosourea: a Phase II-III study of the Eastern Cooperative Oncology Group (EST 4273). Cancer. 1978; 42:2538–2545.20. Lavin P, Mittelman A, Douglass H Jr, Engstrom P, Klaassen D. Survival and response to chemotherapy for advanced colorectal adenocarcinoma: an Eastern Cooperative Oncology Group report. Cancer. 1980; 46:1536–1543.21. Dobrila-Dintinjana R, Trivanovic D, Zelić M, Radić M, Dintinjana M, Petranović D, Toni V, Vukelic J, Matijasic N. Nutritional support in patients with colorectal cancer during chemotherapy: does it work? Hepatogastroenterology. 2013; 60:475–480.22. Taksali SE, Caprio S, Dziura J, Dufour S, Calí AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008; 57:367–371.23. Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008; 88:1263–1271.24. Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010; 95:5419–5426.25. Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997; 5:93–99.26. Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012; 55:2622–2630.