Chonnam Med J.
2020 Jan;56(1):55-61. 10.4068/cmj.2020.56.1.55.
Effect of Low-Dose Nebivolol in Patients with Acute Myocardial Infarction: A Multi-Center Observational Study
- Affiliations
-
- 1Department of Cardiovascular Medicine, Chonnam National University Hospital, Gwanjgu, Korea. myungho@chollian.net
- 2Department of Cardiology, Seoul National University Hospital, Seoul, Korea.
- 3Department of Cardiology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 4Cardiovascular Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Division of Cardiology, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- 6Department of Cardiology, Gachon University Gil Medical Center, Incheon, Korea.
- 7Department of Cardiology, Chungbuk National University Hospital, Cheongju, Korea.
- 8Department of Cardiology, Chungnam National University, Daejeon, Korea.
- 9Division of Cardiology, Yeungnam University Hospital, Daegu, Korea.
- 10Department of Cardiology, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 11Department of Cardiology, Chunbuk National University Hospital, Jeonju, Korea.
- 12Deparment of Cardiology, Wonkwang University Hospital, Iksan, Korea.
- 13Department of Cardiology, Pusan National University Hospital, Busan, Korea.
- 14Department of Cardiology, Kyungsang National University Hospital, Jinju, Korea.
- KMID: 2468147
- DOI: http://doi.org/10.4068/cmj.2020.56.1.55
Abstract
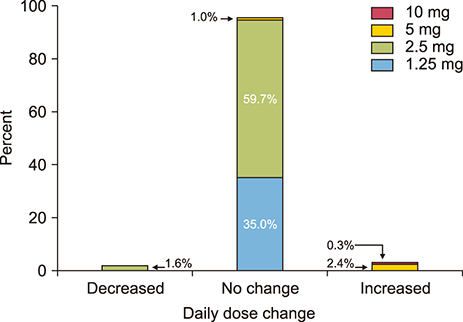
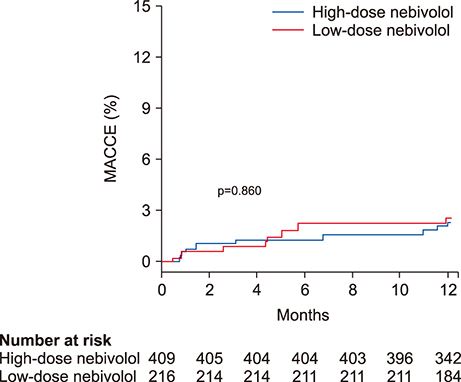
- The optimal dose of beta blockers after acute myocardial infarction (MI) remains uncertain. We evaluated the effectiveness of low-dose nebivolol, a beta1 blocker and a vasodilator, in patients with acute MI. A total of 625 patients with acute MI from 14 teaching hospitals in Korea were divided into 2 groups according to the dose of nebivolol (nebistol®, Elyson Pharmaceutical Co., Ltd., Seoul, Korea): low-dose group (1.25 mg daily, n=219) and usual- to high-dose group (≥2.5 mg daily, n=406). The primary endpoints were major adverse cardiac and cerebrovascular events (MACCE, composite of death from any cause, non-fatal MI, stroke, repeat revascularization, rehospitalization for unstable angina or heart failure) at 12 months. After adjustment using inverse probability of treatment weighting, the rates of MACCE were not different between the low-dose and the usual- to high-dose groups (2.8% and 3.1%, respectively; hazard ratio: 0.92, 95% confidence interval: 0.38 to 2.24, p=0.860). The low-dose nebivolol group showed higher rates of MI than the usual- to high-dose group (1.2% and 0%, p=0.008). The 2 groups had similar rates of death from any cause (1.1% and 0.3%, p=0.273), stroke (0.4% and 1.1%, p=0.384), repeat PCI (1.2% and 0.8%, p=0.428), rehospitalization for unstable angina (1.2% and 1.0%, p=0.743) and for heart failure (0.6% and 0.7%, p=0.832). In patients with acute MI, the rates of MACCE for low-dose and usual- to high-dose nebivolol were not significantly different at 12-month follow-up.
MeSH Terms
Figure
Reference
-
1. Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. First International Study of Infarct Survival Collaborative Group. Lancet. 1986; 2:57–66.2. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985; 27:335–371.
Article3. Kernis SJ, Harjai KJ, Stone GW, Grines LL, Boura JA, O'Neill WW, et al. Does beta-blocker therapy improve clinical outcomes of acute myocardial infarction after successful primary angioplasty? J Am Coll Cardiol. 2004; 43:1773–1779.
Article4. Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, Shimizu M, et al. Osaka Acute Coronary Insufficiency Study (OACIS) Investigators. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am J Cardiol. 2013; 111:457–464.
Article5. Ozasa N, Kimura T, Morimoto T, Hou H, Tamura T, Shizuta S, et al. j-Cypher Registry Investigators. Lack of effect of oral beta-blocker therapy at discharge on long-term clinical outcomes of ST-segment elevation acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol. 2010; 106:1225–1233.
Article6. Yang JH, Hahn JY, Song YB, Choi SH, Choi JH, Lee SH, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2014; 7:592–601.
Article7. Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, et al. OBTAIN Investigators. Effect of beta-blocker dose on survival after acute myocardial infarction. J Am Coll Cardiol. 2015; 66:1431–1441.
Article8. Hwang D, Lee JM, Kim HK, Choi KH, Rhee TM, Park J, et al. KAMIR Investigators. Prognostic impact of β-blocker dose after acute myocardial infarction. Circ J. 2019; 83:410–417.
Article9. Allen JE, Knight S, McCubrey RO, Bair T, Muhlestein JB, Goldberger JJ, et al. β-blocker dosage and outcomes after acute coronary syndrome. Am Heart J. 2017; 184:26–36.
Article10. DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D'Ascenzo F, Cerrato E, et al. β-blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart. 2015; 2:e000230.
Article11. Ripley TL, Saseen JJ. β-blockers: a review of their pharmacological and physiological diversity in hypertension. Ann Pharmacother. 2014; 48:723–733.12. Mrdovic IB, Savic LZ, Perunicic JP, Asanin MR, Lasica RM, Jelena MM, et al. Randomized active-controlled study comparing effects of treatment with carvedilol versus metoprolol in patients with left ventricular dysfunction after acute myocardial infarction. Am Heart J. 2007; 154:116–122.
Article13. Weiss R. Nebivolol: a novel beta-blocker with nitric oxide-induced vasodilatation. Vasc Health Risk Manag. 2006; 2:303–308.
Article14. Münzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol. 2009; 54:1491–1499.15. Chung J, Han JK, Kim YJ, Kim CJ, Ahn Y, Chan Cho M, et al. investigators for Korea Acute Myocardial Infarction Registry (KAMIR). Benefit of vasodilating β-blockers in patients with acute myocardial infarction after percutaneous coronary intervention: nationwide multicenter cohort study. J Am Heart Assoc. 2017; 6:e007063.
Article16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019; 40:237–269.
Article17. Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, et al. SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005; 26:215–225.
Article18. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61:e78–e140.19. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014; 64:e139–e228.20. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–1057.
Article21. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015; 34:3661–3679.
Article22. Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004; 23:2937–2960.
Article23. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008; 168:656–664.
Article24. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014; 33:1242–1258.
Article25. Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981; 304:801–807.26. Hjalmarson A, Herlitz J, Holmberg S, Rydén L, Swedberg K, Vedin A, et al. The Göteborg metoprolol trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation. 1983; 67(6 Pt 2):I26–I32.27. Chadda K, Goldstein S, Byington R, Curb JD. Effect of propranolol after acute myocardial infarction in patients with congestive heart failure. Circulation. 1986; 73:503–510.
Article28. Metoprolol in acute myocardial infarction (MIAMI). A randomized placebo-controlled international trial. The MIAMI Trial Research Group. Eur Heart J. 1985; 6:199–226.29. Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I, et al. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J Am Coll Cardiol. 2011; 57:601–611.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Low Dose Dobutamine Stress Echocardiography in Patients with Acute Myocardial Infarction
- Invasive Treatment of Acute Myocardial Infarction: What is the Optimal Therapy for Acute Myocardial Infarction?
- Effect of Angina Pectoris before Acute Myocardial Infarction on Degree of Residual Stenosis after Successful Coronary Thrombolysis
- Primary Coronary Stenting as a Successful Treatment of Acute Myocardial
- Incidence of Left Ventricular Thrombus after Acute Myocardial Infarction