J Bacteriol Virol.
2019 Dec;49(4):153-161. 10.4167/jbv.2019.49.4.153.
Aralia cordata Extract Activates NF-κB and MAPK Signaling Pathways and Induces Pro-inflammatory Changes in RAW264.7 Macrophages
- Affiliations
-
- 1Gyeongbuk Institute for Bio industry, Andong-si, Gyeongbuk 36618, South Korea.
- 2Department of Animal Science and Technology, Suncheon National University, 255 Jungang-ro, Suncheon-si, Jeollanam-do 57922, South Korea.
- 3Division of Biotechnology, College of Environmental & Bioresources, Jeonbuk National University, Iksan-si, Jeollabukdo 54596, South Korea. leesangm@jbnu.ac.kr
- KMID: 2468022
- DOI: http://doi.org/10.4167/jbv.2019.49.4.153
Abstract
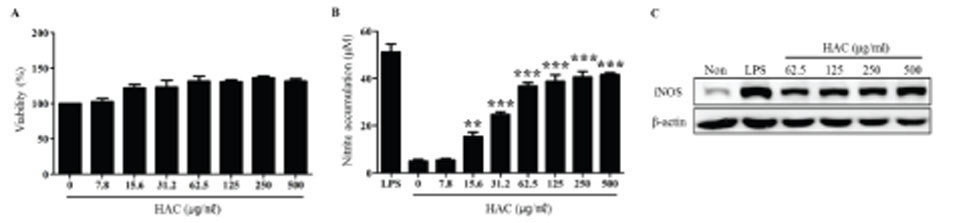
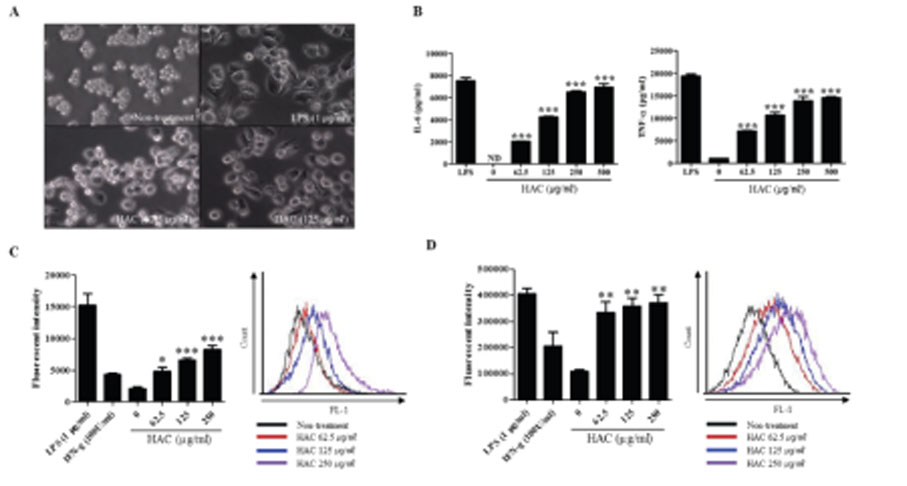
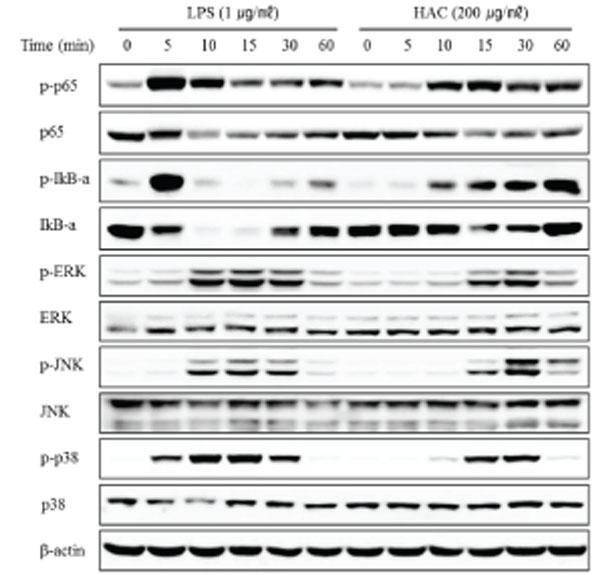
- Macrophages play essential roles in innate immune responses by producing various immune mediators. Therefore, modulating macrophage function is an attractive strategy to treat immune disorders. Aralia cordata var. continentalis (AC), known as "Dokwhal" in Korea, possesses various biological and medicinal functions, including immunomodulation. The present study investigated the effect of the hot water extract of AC (HAC) on RAW264.7 murine macrophages. When these cells were treated with HAC, nitric oxide production and inducible nitric oxide synthase expression was induced dose-dependently. In addition, HAC treatment triggered the secretion of innate immune cytokines, such as TNF-α and IL-6. Phagocytosis, measured by FITC-dextran internalization showed that HAC stimulated the phagocytic activity of macrophages. Furthermore, HAC promoted the production of reactive oxygen species in RAW264.7 cells, determined by CM-H2DCFDA. In addition, the immunoblot analysis of intracellular signaling proteins revealed that NF-kB and MAPK signaling pathways, which are important signaling mediators of inflammation, are upregulated by HAC. In conclusion, these findings suggested that HAC can stimulate macrophage activity, and NF-kB and MAPK signaling pathways might be involved in the immunostimulatory effects of HAC.
Keyword
MeSH Terms
-
Aralia*
Cytokines
Immune System Diseases
Immunity, Innate
Immunomodulation
Inflammation Mediators
Interleukin-6
Intracellular Signaling Peptides and Proteins
Korea
Macrophages*
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Phagocytosis
Reactive Oxygen Species
Water
Cytokines
Inflammation Mediators
Interleukin-6
Intracellular Signaling Peptides and Proteins
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Reactive Oxygen Species
Water
Figure
Reference
-
1. Park CW. The genera of vascular plants of Korea. Seoul: Academic Publishing;2007. p. 1482.2. Perry LM, Metzger J. Medicinal plants of east and southeast Asia: attributed properties and uses MIT press. 1980.3. Hwang YP, Choi JH, Jeong HG. Protective effect of the Aralia continentalis root extract against carbon tetrachlorideinduced hepatotoxicity in mice. Food Chem Toxicol. 2009; 47:75–81.
Article4. Park HJ, Hong MS, Lee JS, Leem KH, Kim CJ, Kim JW, et al. Effects of Aralia continentalis on hyperalgesia with peripheral inflammation. Phytother Res. 2005; 19:511–513.
Article5. Han GJ, Shin DS, Jang MS. A study of the nutritional composition of Aralia continentalis Kitagawa and Aralia continentalis Kitagawa leaf. Korean J Food Sci Technol. 2008; 40:680–685.6. Seo DW, Cho YI, Gu S, Kim DH, Park JH, Yi YJ, et al. A hot water extract of Aralia cordata activates bone marrow-derived macrophages via a myeloid differentiation protein 88-dependent pathway and protects mice from bacterial infection. Microbiol Immunol. 2016; 60:343–355.
Article7. Janeway Jr CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002; 20:197–216.
Article8. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:281–286.
Article9. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001; 2:907–916.
Article10. Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998; 41:1141–1151.
Article11. Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995; 63:736–740.
Article12. Ambs S, Hussain SP, Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997; 11:443–448.
Article13. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25:677–686.
Article14. Lee J, Choi JW, Sohng JK, Pandey RP, Park YI. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-kappaB signaling pathway. Int Immunopharmacol. 2016; 31:88–97.
Article15. Li J, Qian W, Xu Y, Chen G, Wang G, Nie S, et al. Activation of RAW 264.7 cells by a polysaccharide isolated from Antarctic bacterium Pseudoaltermonas sp. S-5. Carbohydr Polym. 2015; 130:97–103.
Article16. Catchpole B, Hamblin AS, Staines NA. Autologous mixed lymphocyte responses in experimentally-induced arthritis of the Lewis rat. Autoimmunity. 2002; 35:111–117.
Article17. Brito LA, Singh M. Acceptable levels of endotoxin in vaccine formulations during preclinical research. J Pharm Sci. 2011; 100:34–37.
Article18. Foresti R, Clark JE, Green CJ, Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J Biol Chem. 1997; 272:18411–18417.
Article19. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999; 17:593–623.
Article20. Kim JS, Kang SS. Saponins from the aerial parts of Aralia continentalis. Nat Prod Sci. 1998; 4:45–50.21. Adachi Y, Okazaki M, Ohno N, Yadomae T. Enhancement of cytokine production by macrophages stimulated with (1-->3)-beta-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol Pharm Bull. 1994; 17:1554–1560.
Article22. Wang ZM, Peng X, Lee KLD, Tang JC, Cheung PCC, Wu JY. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011; 125:637–643.
Article23. Farrell AJ, Blake DR. Nitric oxide. Ann Rheum Dis. 1996; 55:7–20.
Article24. Gorbunov N, Esposito E. Nitric oxide as a mediator of inflammation. Int J Immunopathol Pharmaool. 1993; 6:67–75.25. Yu Q, Nie SP, Li WJ, Zheng WY, Yin PF, Gong DM, et al. Macrophage immunomodulatory activity of a purified polysaccharide isolated from Ganoderma atrum. Phytother Res. 2013; 27:186–191.
Article26. Oppenheim JJ. Cytokines: past, present, and future. Int J Hematol. 2001; 74:3–8.
Article27. Han EH, Choi JH, Hwang YP, Park HJ, Choi CY, Chung YC, et al. Immunostimulatory activity of aqueous extract isolated from Prunella vulgaris. Food Chem Toxicol. 2009; 47:62–69.
Article28. Yue GG, Chan BC, Hon PM, Kennelly EJ, Yeung SK, Cassileth BR, et al. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int J Biol Macromol. 2010; 47:342–347.
Article29. Baeuerle PA, Henkel T. Function and activation of NF-kappaB in the immune system. Annu Rev Immunol. 1994; 12:141–179.
Article30. Lee JY, Kim JY, Lee YG, Rhee MH, Hong EK, Cho JY. Molecular mechanism of macrophage activation by Exopolysaccharides from liquid culture of Lentinus edodes. J Microbiol Biotechnol. 2008; 18:355–364.31. Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994; 269:4705–4708.
Article32. Sun H, Zhang J, Chen F, Chen X, Zhou Z, Wang H. Activation of RAW264. 7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr Polym. 2015; 121:388–402.
Article33. Kim YS, Kim EK, Nawarathna WPAS, Dong X, Shin WB, Park JS, et al. Immune-Stimulatory Effects of Althaea rosea Flower Extracts through the MAPK Signaling Pathway in RAW264.7 Cells. Molecules. 2017; 22.
Article34. Bai Y, Jiang Y, Liu T, Li F, Zhang J, Luo Y, et al. Xinjiang herbal tea exerts immunomodulatory activity via TLR2/4-mediated MAPK signaling pathways in RAW264.7 cells and prevents cyclophosphamide-induced immunosuppression in mice. J Ethnopharmacol. 2019; 228:179–187.
Article35. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912.
Article36. Rao KM. MAP kinase activation in macrophages. J Leukoc Biol. 2001; 69:3–10.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Anti-Inflammatory Effect of Mangostenone F in Lipopolysaccharide-Stimulated RAW264.7 Macrophages by Suppressing NF-kappaB and MAPK Activation
- Aloe-emodin inhibits Pam₃CSK₄-induced MAPK and NF-κB signaling through TLR2 in macrophages
- Immunomodulating activity of Sargassum horneri extracts in RAW264.7 macrophages
- Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-kappaB, and MAPK Pathways