Transl Clin Pharmacol.
2019 Dec;27(4):123-126. 10.12793/tcp.2019.27.4.123.
Treatment response and disease progression
- Affiliations
-
- 1Department of Pharmacology & Clinical Pharmacology, University of Auckland, Auckland, New Zealand. n.holford@auckland.ac.nz
- KMID: 2467965
- DOI: http://doi.org/10.12793/tcp.2019.27.4.123
Abstract
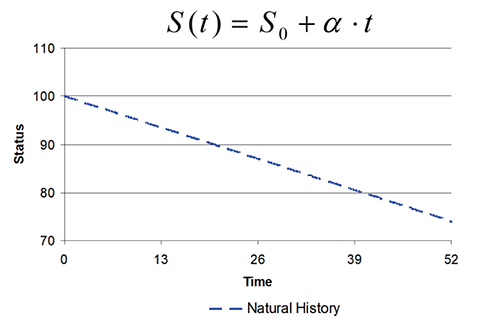
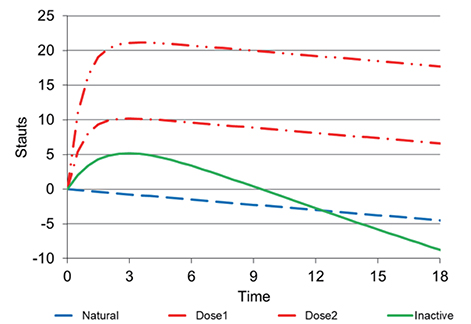
- This tutorial defines the concepts of disease progression in the context of clinical pharmacology. Disease progression describes the natural history of disease, such as pain, or biomarker of drug response, such as blood pressure. The action of a drug, such as inhibiting an enzyme or activating a receptor, leads to a change in disease status over time. Two main types of drug response can be defined based on the pattern of the time course of disease status. The most common is a symptomatic effect equivalent to a shift up or down of the natural history curve. Less common but quite clinically important is a disease-modifying effect equivalent to a change in the rate of disease progression.
Figure
Reference
-
1. Holford N. Pharmacodynamic principles and the time course of immediate drug effects. Transl Clin Pharmacol. 2017; 25:157–161.
Article2. Holford N. Absorption and Half-Life. Transl Clin Pharmacol. 2016; 24:157–160.
Article3. Holford N, Yim D-S. Clearance. Transl Clin Pharmacol. 2015; 23:42–45.
Article4. Holford N, Yim D-S. Volume of distribution. Transl Clin Pharmacol. 2016; 24:74–77.
Article5. Bolland MJ, Grey A, Reid IR. Should we prescribe calcium or vitamin D supplements to treat or prevent osteoporosis? Climacteric. 2015; 18 Suppl 2:22–31. DOI: 10.3109/13697137.2015.1098266.
Article6. Holford NH, Chan PL, Nutt JG, Kieburtz K, Shoulson I. Disease progression and pharmacodynamics in Parkinson disease - evidence for functional protection with levodopa and other treatments. J Pharmacokinet Pharmacodyn. 2006; 33:281–311.
Article7. Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med. 1989; 321:1364–1371.8. Griggs RC, Moxley RT 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne Dystrophy: A randomized, controlled trial defining the time course and dose response. Arch Neurol. 1991; 48:383–388.9. Holford NH, Peace K. The effect of tacrine and lecithin in Alzheimer's disease. A population pharmacodynamic analysis of five clinical trials. Eur J Clin Pharmacol. 1994; 47:17–23.10. Holford NH, Peace KE. Results and validation of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc Natl Acad Sci U S A. 1992; 89:11471–11475.
Article11. Imbimbo BP, Verdelli G, Martelli P, Marchesini D. The Eptastigmine Study Group. Two-year treatment of Alzheimer's disease with eptastigmine. Dement Geriatr Cogn Disord. 1999; 10:139–147.
Article12. Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson's disease and their response to treatment. Br J Clin Pharmacol. 2012; 74:267–283. DOI: 10.1111/j.1365-2125.2012.04192.x.
Article13. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-Year Trial of Tiotropium in Chronic Obstructive Pulmonary Disease. N Engl J Med. 2008; 359:1543–1554. DOI: 10.1056/NEJMoa0805800.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Prognostic Factors of Patients with Superficial Bladder Cancer to a Second Course of Intravesical Bacillus Calmette-Guerin
- Role of radiotherapy for pancreatobiliary neuroendocrine tumors
- Results and Indication of Patients with Superficial Bladder Cancer to a Second Course of Intravesical Bacillus Calmette-Guerin
- Treatment of Langerhans Cell Histiocytosis with Indomethacin
- Progression of Peyronie's Disease during Tamoxifen Treatment