J Clin Neurol.
2020 Jan;16(1):29-36. 10.3988/jcn.2020.16.1.29.
Plasma Fibroblast Growth Factor 23 Concentration Is Associated with Intracranial Cerebral Atherosclerosis in Acute Ischemic Stroke Patients
- Affiliations
-
- 1Department of Neurology, Ewha Womans University Mokdong Hospital, Ewha Womans University College of Medicine, Seoul, Korea. knstar@ewha.ac.kr
- 2Department of Neurology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Ewha Womans University Seoul Hospital, Ewha Womans University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea.
- 5Ewha Institute of Convergence Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- KMID: 2467781
- DOI: http://doi.org/10.3988/jcn.2020.16.1.29
Abstract
- BACKGROUND AND PURPOSE
Fibroblast growth factor 23 (FGF23) is associated with atherosclerosis via nitric-oxide-associated endothelial dysfunction and calcium-phosphate-related bone mineralization. This study aimed to determine the association of the plasma FGF23 concentration with intracranial cerebral atherosclerosis (ICAS) and extracranial cerebral atherosclerosis (ECAS).
METHODS
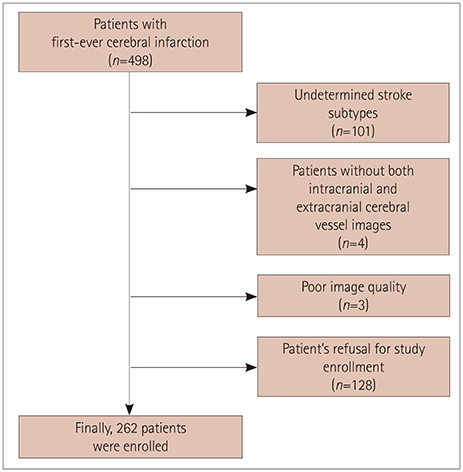
We prospectively enrolled 262 first-ever ischemic stroke patients in whom brain magnetic resonance was performed and a blood sample acquired within 24 h after admission. Plasma FGF23 concentrations were measured using an enzyme-linked immunosorbent assay. The presence of ICAS or ECAS was defined as a ≥50% decrease in arterial diameter in magnetic resonance angiography. The burden of cerebral atherosclerosis was calculated by adding the total number of vessels defined as ICAS or ECAS.
RESULTS
Our study population included 152 (58.0%) males. The mean age was 64.7 years, and the plasma FGF23 concentration was 347.5±549.6 pg/mL (mean±SD). ICAS only, ECAS only, and both ICAS and ECAS were present in 31.2% (n=82), 4.9% (n=13), and 6.8% (n=18) of the subjects, respectively. In multivariate binary and ordinal logistic analyses, after adjusting for sex, age, and variables for which p < 0.1 in the univariate analysis, the plasma FGF23 concentration (per 100 pg/mL) was positively correlated with the presence of ICAS [odds ratio (OR)=1.07, 95% CI=1.00-1.15, p=0.039], burden of ICAS (OR=1.09, 95% CI=1.04-1.15, p=0.001), and burden of ECAS (OR=1.06, 95% CI=1.00-1.12, p=0.038), but it was not significantly related to the presence of ECAS (OR=1.05, 95% CI=0.99-1.12, p=0.073).
CONCLUSIONS
The plasma FGF23 may be a potential biomarker for cerebral atherosclerosis, particularly the presence and burden of ICAS in stroke patients.
MeSH Terms
Figure
Reference
-
1. Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014; 16:27–35.
Article2. Arenillas JF, Molina CA, Chacón P, Rovira A, Montaner J, Coscojuela P, et al. High lipoprotein (a), diabetes, and the extent of symptomatic intracranial atherosclerosis. Neurology. 2004; 63:27–32.
Article3. Park KY, Chung CS, Lee KH, Kim GM, Kim YB, Oh K. Prevalence and risk factors of intracranial atherosclerosis in an asymptomatic Korean population. J Clin Neurol. 2006; 2:29–33.
Article4. Song TJ, Park JH, Choi KH, Kim JH, Choi Y, Chang Y, et al. Is obstructive sleep apnea associated with the presence of intracranial cerebral atherosclerosis. Sleep Breath. 2017; 21:639–646.
Article5. ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000; 26:345–348.6. Martin A, David V, Quarles LD. Regulation and function of the FGF23/Klotho endocrine pathways. Physiol Rev. 2012; 92:131–155.
Article7. Zoccali C, Yilmaz MI, Mallamaci F. FGF23: a mature renal and cardiovascular risk factor? Blood Purif. 2013; 36:52–57.
Article8. Donate-Correa J, Muros de, Mora-Fernández C, Navarro-González JF. Pathophysiological implications of fibroblast growth factor-23 and Klotho and their potential role as clinical biomarkers. Clin Chem. 2014; 60:933–940.
Article9. Hu X, Ma X, Luo Y, Xu Y, Xiong Q, Pan X, et al. Elevation in fibroblast growth factor 23 and its value for identifying subclinical atherosclerosis in first-degree relatives of patients with diabetes. Sci Rep. 2016; 6:34696.
Article10. Shibata K, Fujita S, Morita H, Okamoto Y, Sohmiya K, Hoshiga M, et al. Association between circulating fibroblast growth factor 23, α-Klotho, and the left ventricular ejection fraction and left ventricular mass in cardiology inpatients. PLoS One. 2013; 8:e73184.
Article11. Malyszko J, Koc-Zorawska E, Matuszkiewicz-Rowinska J, Malyszko J. FGF23 and Klotho in relation to markers of endothelial dysfunction in kidney transplant recipients. Transplant Proc. 2014; 46:2647–2650.
Article12. Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, et al. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin J Am Soc Nephrol. 2013; 8:781–786.
Article13. Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, et al. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012; 97:E575–E583.
Article14. Song TJ, Kim YD, Yoo J, Kim J, Chang HJ, Hong GR, et al. Association between aortic atheroma and cerebral small vessel disease in patients with ischemic stroke. J Stroke. 2016; 18:312–320.
Article15. Chang Y, Kim J, Kim MH, Kim YJ, Song TJ. Interarm blood pressure difference is associated with early neurological deterioration, poor short-term functional outcome, and mortality in noncardioembolic stroke patients. J Clin Neurol. 2018; 14:555–565.
Article16. Song TJ, Chang Y, Chun MY, Lee CY, Kim AR, Kim Y, et al. High dietary glycemic load is associated with poor functional outcome in patients with acute cerebral infarction. J Clin Neurol. 2018; 14:165–173.
Article17. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
Article18. Song TJ, Kim J, Yang SH, Park JH, Lee HS, Nam CM, et al. Association of plasma osteoprotegerin levels with stroke severity and functional outcome in acute ischaemic stroke patients. Biomarkers. 2012; 17:738–744.
Article19. Song TJ, Kim J, Kim YD, Nam HS, Lee HS, Nam CM, et al. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. Eur J Neurol. 2014; 21:463–469.
Article20. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247–254.
Article21. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000; 21:643–646.22. Kim J, Cha MJ, Lee DH, Lee HS, Nam CM, Nam HS, et al. The association between cerebral atherosclerosis and arterial stiffness in acute ischemic stroke. Atherosclerosis. 2011; 219:887–891.
Article23. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453.
Article24. Chang Y, Choi GS, Lim SM, Kim YJ, Song TJ. Interarm systolic and diastolic blood pressure difference is diversely associated with cerebral atherosclerosis in noncardioembolic stroke patients. Am J Hypertens. 2017; 31:35–42.
Article25. Lima F, El-Husseini A, Monier-Faugere MC, David V, Mawad H, Quarles D, et al. FGF-23 serum levels and bone histomorphometric results in adult patients with chronic kidney disease on dialysis. Clin Nephrol. 2014; 82:287–295.
Article26. Mirza MA, Hansen T, Johansson L, Ahlström H, Larsson A, Lind L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009; 24:3125–3131.
Article27. Semba RD, Fink JC, Sun K, Cappola AR, Dalal M, Crasto C, et al. Serum fibroblast growth factor-23 and risk of incident chronic kidney disease in older community-dwelling women. Clin J Am Soc Nephrol. 2012; 7:85–91.
Article28. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011; 121:4393–4408.
Article29. Kanbay M, Nicoleta M, Selcoki Y, Ikizek M, Aydin M, Eryonucu B, et al. Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin J Am Soc Nephrol. 2010; 5:1780–1786.
Article30. Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016; 90:985–996.
Article31. Xie D, Deng L, Liu XD, Li JM, Zhang YB. Role of high sensitivity C-reactive protein and other risk factors in intracranial and extracranial artery occlusion in patients with ischaemic stroke. J Int Med Res. 2015; 43:711–717.
Article32. Lanske B, Razzaque MS. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 2014; 86:1072–1074.
Article33. Rodriguez-Ortiz ME, Lopez I, Muñoz-Castañeda JR, Martinez-Moreno JM, Ramírez AP, Pineda C, et al. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012; 23:1190–1197.
Article34. Kang K. Serum calcium and phosphate concentrations and intracranial atherosclerosis. Atherosclerosis. 2014; 232:249–253.
Article35. Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009; 20:381–387.
Article36. Pini R, Faggioli G, Fittipaldi S, Vasuri F, Longhi M, Gallitto E, et al. Relationship between calcification and vulnerability of the carotid plaques. Ann Vasc Surg. 2017; 44:336–342.
Article37. Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 2009; 205:385–390.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasma Levels of Soluble Adhesion Molecules in Patients with Acute Cerebral Ischemic Stroke
- Association of Metabolic Syndrome and C-reactive Protein Levels with Intracranial Atherosclerotic Stroke
- Acute Ischemic Stroke Associated with Essential Thrombocythemia and Suspected Platelet Aggregation in Intracranial Artery
- Impact of Intracranial Cerebral Atherosclerosis on the Long-term Mortality after Ischemic Stroke
- Relations to Plasma Fibrinogen Concentration and Subtype, Prognostic Influence in Patients with Acute Ischemic Stroke