J Clin Neurol.
2019 Apr;15(2):159-167. 10.3988/jcn.2019.15.2.159.
Interarm Blood Pressure Difference has Various Associations with the Presence and Burden of Cerebral Small-Vessel Diseases in Noncardioembolic Stroke Patients
- Affiliations
-
- 1Department of Neurology, College of Medicine, Ewha Womans University, Seoul, Korea. knstar@ewha.ac.kr
- 2Departent of Neurology, College of Medicine, Korea University Guro Hostpital, Seoul, Korea.
- KMID: 2467729
- DOI: http://doi.org/10.3988/jcn.2019.15.2.159
Abstract
- BACKGROUND AND PURPOSE
An interarm blood pressure difference (IABD) is independently related to the occurrence of cardiovascular disease and mortality. Cerebral small-vessel diseases (SVDs) are important risk factors for stroke, cognitive dysfunction, and mortality. We aimed to determine whether IABD is related to cerebral SVDs.
METHODS
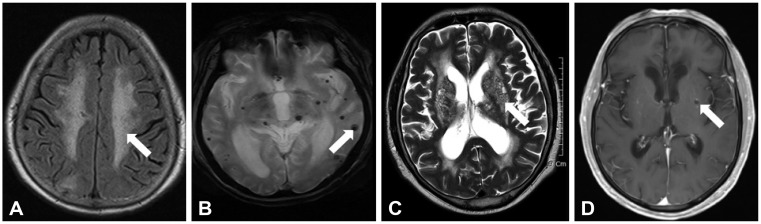
This study included 1,205 consecutive noncardioembolic ischemic stroke patients as confirmed by brain MRI and simultaneously measured the bilateral brachial blood pressures. We investigated cerebral SVDs based on high-grade white-matter hyperintensities (HWHs), presence of cerebral microbleeds (CMBs), high-grade perivascular spaces (HPVSs), and asymptomatic lacunar infarctions (ALIs) on brain MRI.
RESULTS
In multivariate logistic regression, an interarm systolic blood pressure difference (IASBD) ≥10 mm Hg was independently related to the existence of HWHs [odds ratio (OR)=1.94, 95% CI=1.32-2.84, p=0.011] and had a tendency to be associated with the presence of HPVSs (OR=1.45, 95% CI=0.49-2.23, p=0.089) and ALIs (OR=1.42, 95% CI=0.96-2.11, p=0.052), but not with the presence of CMBs (OR=1.09, 95% CI=0.73-1.61, p=0.634). In multivariate linear regression adjusted for age, sex, and variables with p<0.1 in the univariate analysis, IASBD ≥10 mm Hg and interarm diastolic blood pressure difference ≥10 mm Hg were significantly correlated with an increased total burden of SVDs (β=0.080 and p=0.006, and β=0.065 and p=0.023, respectively).
CONCLUSIONS
This study found that IABD ≥10 mm Hg was associated with the presence and increased burden of cerebral SVDs in noncardioembolic stroke patients. This suggests that IABD ≥10 mm Hg could be a useful indicator of the presence and burden of cerebral SVDs in stroke patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Clark CE, Taylor RS, Shore AC, Campbell JL. Prevalence of systolic inter-arm differences in blood pressure for different primary care populations: systematic review and meta-analysis. Br J Gen Pract. 2016; 66:e838–e847. PMID: 27789511.
Article2. Kim J, Song TJ, Song D, Lee HS, Nam CM, Nam HS, et al. Interarm blood pressure difference and mortality in patients with acute ischemic stroke. Neurology. 2013; 80:1457–1464. PMID: 23516316.
Article3. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012; 379:905–914. PMID: 22293369.
Article4. Aboyans V, Criqui MH, McDermott MM, Allison MA, Denenberg JO, Shadman R, et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007; 49:1540–1545. PMID: 17418292.
Article5. Han F, Zhai FF, Wang Q, Zhou LX, Ni J, Yao M, et al. Prevalence and risk factors of cerebral small vessel disease in a Chinese population-based sample. J Stroke. 2018; 20:239–246. PMID: 29886722.
Article6. Song TJ, Kim J, Song D, Nam HS, Kim YD, Lee HS, et al. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology. 2014; 83:1308–1315. PMID: 25186853.
Article7. Tsai HH, Kim JS, Jouvent E, Gurol ME. Updates on prevention of hemorrhagic and lacunar strokes. J Stroke. 2018; 20:167–179. PMID: 29886717.
Article8. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010; 41:450–454. PMID: 20056930.
Article9. Kim BJ, Lee SH. Cerebral microbleeds: their associated factors, radiologic findings, and clinical implications. J Stroke. 2013; 15:153–163. PMID: 24396809.
Article10. Song TJ, Kim J, Song D, Yoo J, Lee HS, Kim YJ, et al. Total cerebral small-vessel disease score is associated with mortality during follow-up after acute ischemic stroke. J Clin Neurol. 2017; 13:187–195. PMID: 28406586.
Article11. Ochoa VM, Yeghiazarians Y. Subclavian artery stenosis: a review for the vascular medicine practitioner. Vasc Med. 2011; 16:29–34. PMID: 21078767.
Article12. Canepa M, Milaneschi Y, Ameri P, AlGhatrif M, Leoncini G, Spallarossa P, et al. Relationship between inter-arm difference in systolic blood pressure and arterial stiffness in community-dwelling older adults. J Clin Hypertens (Greenwich). 2013; 15:880–887. PMID: 24299691.
Article13. Song TJ, Cho HJ, Chang Y, Choi K, Jung AR, Youn M, et al. Low plasma proportion of omega 3-polyunsaturated fatty acids predicts poor outcome in acute non-cardiogenic ischemic stroke patients. J Stroke. 2015; 17:168–176. PMID: 26060804.
Article14. Chang Y, Kim J, Kim MH, Kim YJ, Song TJ. Interarm blood pressure difference is associated with early neurological deterioration, poor short-term functional outcome, and mortality in noncardioembolic stroke patients. J Clin Neurol. 2018; 14:555–565. PMID: 30284767.
Article15. Song TJ, Kim J, Kim YD, Nam HS, Lee HS, Nam CM, et al. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. Eur J Neurol. 2014; 21:463–469. PMID: 24330330.
Article16. Motobe K, Tomiyama H, Koji Y, Yambe M, Gulinisa Z, Arai T, et al. Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J. 2005; 69:55–60. PMID: 15635203.
Article17. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. PMID: 7678184.
Article18. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002; 25:359–364. PMID: 12135313.
Article19. Verberk WJ, Kessels AG, Thien T. Blood pressure measurement method and inter-arm differences: a meta-analysis. Am J Hypertens. 2011; 24:1201–1208. PMID: 21776035.
Article20. Moon J, Choi KH, Park JH, Song TJ, Choi YS, Kim JH, et al. Sympathetic overactivity based on heart-rate variability in patients with obstructive sleep apnea and cerebral small-vessel disease. J Clin Neurol. 2018; 14:310–319. PMID: 29856154.
Article21. Song TJ, Park JH, Choi KH, Chang Y, Moon J, Kim JH, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017; 30:36–42. PMID: 28215260.
Article22. Song TJ, Kim J, Lee HS, Nam CM, Nam HS, Kim EH, et al. Differential impact of unrecognised brain infarction on stroke outcome in non-valvular atrial fibrillation. Thromb Haemost. 2014; 112:1312–1318. PMID: 25231184.
Article23. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12:822–838. PMID: 23867200.
Article24. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014; 83:1228–1234. PMID: 25165388.
Article25. Song TJ, Kim YD, Yoo J, Kim J, Chang HJ, Hong GR, et al. Association between aortic atheroma and cerebral small vessel disease in patients with ischemic stroke. J Stroke. 2016; 18:312–320. PMID: 27488980.
Article26. Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Guerrieri M, Zampi I, et al. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol. 1994; 74:714–719. PMID: 7942532.
Article27. Pase MP, Beiser A, Aparicio H, DeCarli C, Vasan RS, Murabito J, et al. Interarm differences in systolic blood pressure and the risk of dementia and subclinical brain injury. Alzheimers Dement. 2016; 12:438–445. PMID: 26542262.
Article28. Arba F, Mair G, Carpenter T, Sakka E, Sandercock PAG, Lindley RI, et al. Cerebral white matter hypoperfusion increases with small-vessel disease burden. Data from the third international stroke trial. J Stroke Cerebrovasc Dis. 2017; 26:1506–1513. PMID: 28314624.
Article29. Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord. 1998; 9(Suppl 1):2–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interarm Blood Pressure Difference is Associated with Early Neurological Deterioration, Poor Short-Term Functional Outcome, and Mortality in Noncardioembolic Stroke Patients
- Vascular Overload Index Predicts Cerebral Small Vessel Disease in a Neurologically Healthy Population
- Cerebral Small Vessel Disease and Chronic Kidney Disease
- Prognostic Impact of Cerebral Small Vessel Disease on Stroke Outcome
- Prevention and Management of Cerebral Small Vessel Disease