Ann Dermatol.
2020 Feb;32(1):21-30. 10.5021/ad.2020.32.1.21.
Minocycline and Its Impact on Microbial Dysbiosis in the Skin and Gastrointestinal Tract of Acne Patients
- Affiliations
-
- 1Department of Dermatology, Johns Hopkins University, Baltimore, MD, USA. achien3@jhmi.edu
- 2Department of Dermatology, Medical University of Graz, Graz, Austria.
- 3Institute of Genetic Medicine, Johns Hopkins University, MD, USA.
- 4Institute for Genome Sciences, University of Maryland, Baltimore, MD, USA.
- KMID: 2467197
- DOI: http://doi.org/10.5021/ad.2020.32.1.21
Abstract
- BACKGROUND
Associations between acne and gastrointestinal comorbidities suggest that microbial dysbiosis and intestinal permeability may promote inflammatory acne, a condition often managed with oral antibiotics.
OBJECTIVE
We performed a case-control study to investigate the skin and gut microbiota in 8 acne patients before and after receiving oral minocycline compared to controls matched by age ±5 years, sex, and race.
METHODS
DNA was extracted from stool samples and facial skin swabs. Sequencing of the V3V4 region of the bacterial 16S rRNA gene was performed using Illumina MiSeq and analyzed using QIIME/MetaStats 2.0 software.
RESULTS
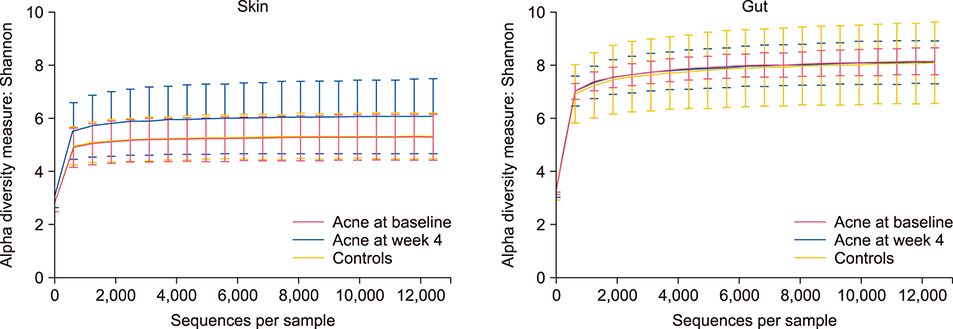
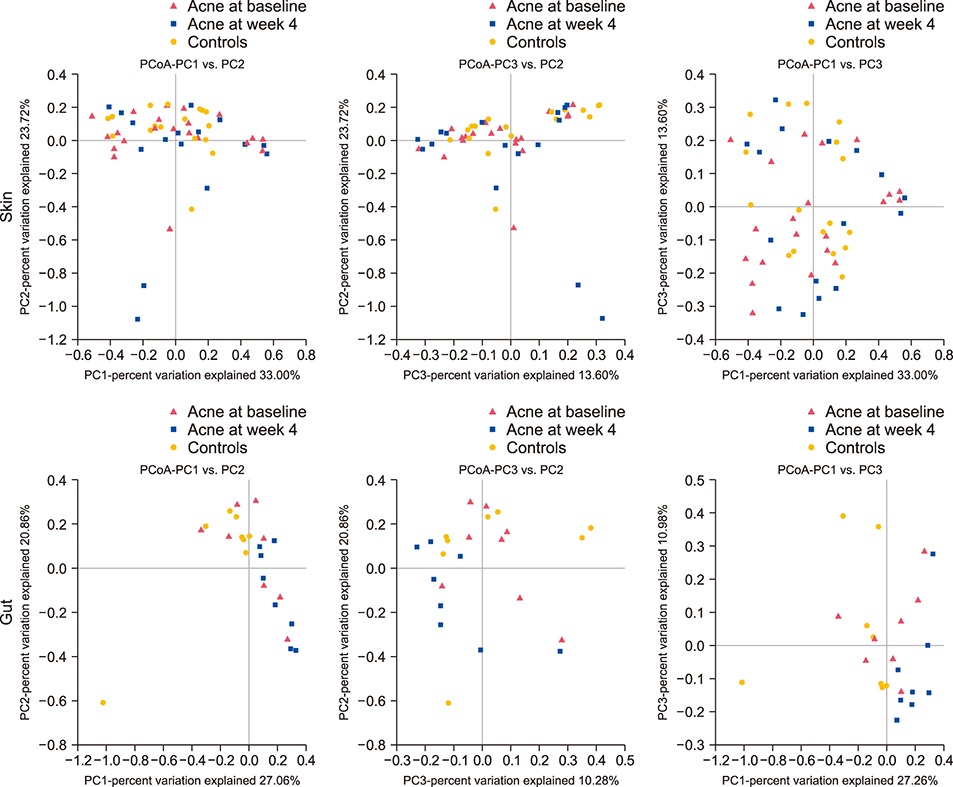
Acne patients included 7 female and 1 male, ages 20~32. Shannon diversity was not significantly different between the skin (p=0.153) or gut (p < 0.999) microbiota of acne patients before and after antibiotics. The gut microbiota in pre-antibiotic acne patients compared to acne-free controls was depleted in probiotics Lactobacillus iners (p=0.001), Lactobacillus zeae (p=0.001), and Bifidobacterium animalis (p=0.026). After antibiotics, the gut microbiota of acne patients was depleted in Lactobacillus salivarius (p=0.001), Bifidobacterium adolescentis (p=0.002), Bifidobacterium pseudolongum (p=0.010), and Bifidobacterium breve (p=0.042), while the skin microbiota was enriched in probiotics Bifidobacterium longum (p=0.028) and Leuconostoc mesenteroides (p=0.029) and depleted in Staphylococcus epidermidis (p=0.009) and Prevotella nigrescens (p=0.028). At the phylum level, significant enrichment of Bacteroidetes in stool of acne patients following antibiotic treatment (p=0.033) led to a decreased Firmicutes to Bacteroidetes ratio.
CONCLUSION
Minocycline produces significant derangements in the microbiota of the skin and gut, including many probiotic species, highlighting the potential for more targeted antimicrobial treatments for acne.
MeSH Terms
-
Acne Vulgaris*
Anti-Bacterial Agents
Bacteroidetes
Bifidobacterium
Case-Control Studies
Comorbidity
Continental Population Groups
DNA
Dysbiosis*
Female
Firmicutes
Gastrointestinal Microbiome
Gastrointestinal Tract*
Genes, rRNA
Humans
Lactobacillus
Leuconostoc
Male
Microbiota
Minocycline*
Permeability
Prevotella nigrescens
Probiotics
Propionibacterium
Skin*
Staphylococcus epidermidis
Sulfalene
Anti-Bacterial Agents
DNA
Minocycline
Sulfalene
Figure
Reference
-
1. Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015; 60:1308–1316.
Article2. Del Rosso JQ, Webster GF, Rosen T, Thiboutot D, Leyden JJ, Gallo R, et al. Status report from the scientific panel on antibiotic use in dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016; 9:18–24.3. Coates P, Vyakrnam S, Eady EA, Jones CE, Cove JH, Cunliffe WJ. Prevalence of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol. 2002; 146:840–848.
Article4. Ross JI, Snelling AM, Carnegie E, Coates P, Cunliffe WJ, Bettoli V, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003; 148:467–478.
Article5. Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005; 153:395–403.
Article6. Levy RM, Huang EY, Roling D, Leyden JJ, Margolis DJ. Effect of antibiotics on the oropharyngeal flora in patients with acne. Arch Dermatol. 2003; 139:467–471.
Article7. Mills O Jr, Thornsberry C, Cardin CW, Smiles KA, Leyden JJ. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol. 2002; 82:260–265.
Article8. Zhang H, Liao W, Chao W, Chen Q, Zeng H, Wu C, et al. Risk factors for sebaceous gland diseases and their relationship to gastrointestinal dysfunction in Han adolescents. J Dermatol. 2008; 35:555–561.
Article9. Stokes JH, Pillsbury DM. The effect on the skin of emotional and nervous states Iii. Theoretical and practical consideration of a gastro-intestinal mechanism. Arch Derm Syphilol. 1930; 22:962–993.
Article10. Lauritano EC, Valenza V, Sparano L, Scarpellini E, Gabrielli M, Cazzato A, et al. Small intestinal bacterial overgrowth and intestinal permeability. Scand J Gastroenterol. 2010; 45:1131–1132.
Article11. Juhlin L, Michaëlsson G. Fibrin microclot formation in patients with acne. Acta Derm Venereol. 1983; 63:538–540.12. Terhorst D, Kalali BN, Ollert M, Ring J, Mempel M. The role of toll-like receptors in host defenses and their relevance to dermatologic diseases. Am J Clin Dermatol. 2010; 11:1–10.
Article13. Deng Y, Wang H, Zhou J, Mou Y, Wang G, Xiong X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol. 2018; 98:783–790.
Article14. Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013; 98:111–120.
Article15. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012; 486:222–227.
Article16. Obregon-Tito AJ, Tito RY, Metcalf J, Sankaranarayanan K, Clemente JC, Ursell LK, et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat Commun. 2015; 6:6505.
Article17. Gomez A, Petrzelkova KJ, Burns MB, Yeoman CJ, Amato KR, Vlckova K, et al. Gut microbiome of coexisting BaAka pygmies and bantu reflects gradients of traditional subsistence patterns. Cell Rep. 2016; 14:2142–2153.
Article18. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010; 107:14691–14696.
Article19. O'Brien SC, Lewis JB, Cunliffe WJ. The Leeds revised acne grading system. J Dermatolog Treat. 1998; 9:215–220.20. Roghmann MC, Lydecker AD, Hittle L, DeBoy RT, Nowak RG, Johnson JK, et al. Comparison of the microbiota of older adults living in nursing homes and the community. mSphere. 2017; 2:e00210–e00217.
Article21. Bromberg JS, Hittle L, Xiong Y, Saxena V, Smyth EM, Li L, et al. Gut microbiota-dependent modulation of innate immunity and lymph node remodeling affects cardiac allograft outcomes. JCI Insight. 2018; 3:121045.
Article22. Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014; 2:6.
Article23. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7:335–336.
Article24. White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009; 5:e1000352.
Article25. Clarke KR. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993; 18:117–143.
Article26. Loveman DE, Noojin RO, Winkler CH Jr. Comparative studies of enteric bacterial flora in acne vulgaris. J Invest Dermatol. 1955; 25:135–137.27. Chien AL, Tsai J, Leung S, Mongodin EF, Nelson AM, Kang S, et al. Association of systemic antibiotic treatment of acne with skin microbiota characteristics. JAMA Dermatol. 2019; 155:425–434.
Article28. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011; 469:543–547.
Article29. Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A doubleblind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017; 51:810–821.
Article30. Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016; 6:e939.
Article31. Pujato SA, Guglielmotti DM, Martínez-García M, Quiberoni A, Mojica FJM. Leuconostoc mesenteroides and Leuconostoc pseudomesenteroides bacteriophages: Genomics and crossspecies host ranges. Int J Food Microbiol. 2017; 257:128–137.
Article32. Matsuzaki C, Takagaki C, Tomabechi Y, Forsberg LS, Heiss C, Azadi P, et al. Structural characterization of the immunostimulatory exopolysaccharide produced by Leuconostoc mesenteroides strain NTM048. Carbohydr Res. 2017; 448:95–102.
Article33. Zununi Vahed S, Barzegari A, Rahbar Saadat Y, Goreyshi A, Omidi Y. Leuconostoc mesenteroides-derived anticancer pharmaceuticals hinder inflammation and cell survival in colon cancer cells by modulating NF-κB/AKT/PTEN/MAPK pathways. Biomed Pharmacother. 2017; 94:1094–1100.
Article34. Wang YY, Ryu AR, Jin S, Jeon YM, Lee MY. Chlorin e6-mediated photodynamic therapy suppresses P. acnes-induced inflammatory response via NFκB and MAPKs signaling pathway. PLoS One. 2017; 12:e0170599.
Article35. Chen Q, Koga T, Uchi H, Hara H, Terao H, Moroi Y, et al. Propionibacterium acnes-induced IL-8 production may be mediated by NF-kappaB activation in human monocytes. J Dermatol Sci. 2002; 29:97–103.
Article36. Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol. 2014; 98:411–424.
Article37. Xia X, Li Z, Liu K, Wu Y, Jiang D, Lai Y. Staphylococcal LTA-induced miR-143 inhibits propionibacterium acnes-mediated inflammatory response in skin. J Invest Dermatol. 2016; 136:621–630.
Article38. Lie MA, van der, Timmerman MF, Loos BG, van Steenbergen TJ, van der Velden U. Occurrence of Prevotella intermedia and Prevotella nigrescens in relation to gingivitis and gingival health. J Clin Periodontol. 2001; 28:189–193.
Article39. Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007; 57:247–256.
Article40. Falsen E, Pascual C, Sjödén B, Ohlén M, Collins MD. Phenotypic and phylogenetic characterization of a novel Lactobacillus species from human sources: description of Lactobacillus iners sp. nov. Int J Syst Bacteriol. 1999; 49 Pt 1:217–221.
Article41. Zhou M, Yu H, Yin X, Sabour PM, Chen W, Gong J. Lactobacillus zeae protects Caenorhabditis elegans from enterotoxigenic Escherichia coli-caused death by inhibiting enterotoxin gene expression of the pathogen. PLoS One. 2014; 9:e89004.
Article42. Jungersen M, Wind A, Johansen E, Christensen JE, Stuer-Lauridsen B, Eskesen D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12(®). Microorganisms. 2014; 2:92–110.
Article43. Messaoudi S, Manai M, Kergourlay G, Prévost H, Connil N, Chobert JM, et al. Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol. 2013; 36:296–304.
Article44. O'Shea EF, O'Connor PM, Raftis EJ, O'Toole PW, Stanton C, Cotter PD, et al. Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. J Bacteriol. 2011; 193:6973–6982.45. Lee J, Ametani A, Enomoto A, Sato Y, Motoshima H, Ike F, et al. Screening for the immunopotentiating activity of food microorganisms and enhancement of the immune response by bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem. 1993; 57:2127–2132.
Article46. Li D, Breiman A, le Pendu J, Uyttendaele M. Anti-viral Effect of Bifidobacterium adolescentis against noroviruses. Front Microbiol. 2016; 7:864.
Article47. An HM, Lee DK, Kim JR, Lee SW, Cha MK, Lee KO, et al. Antiviral activity of Bifidobacterium adolescentis SPM 0214 against herpes simplex virus type 1. Arch Pharm Res. 2012; 35:1665–1671.
Article48. Cha MK, Lee DK, An HM, Lee SW, Shin SH, Kwon JH, et al. Antiviral activity of Bifidobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Med. 2012; 10:72.
Article49. Kim MJ, Lee DK, Park JE, Park IH, Seo JG, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM1605 against Coxsackievirus B3. Biotechnol Biotechnol Equip. 2014; 28:681–688.
Article50. Mangin I, Dossou-Yovo F, Lévêque C, Dessoy MV, Sawoo O, Suau A, et al. Oral administration of viable Bifidobacterium pseudolongum strain Patronus modified colonic microbiota and increased mucus layer thickness in rat. FEMS Microbiol Ecol. 2018; 94(11):
Article51. Pélissier MA, Vasquez N, Balamurugan R, Pereira E, Dossou-Yovo F, Suau A, et al. Metronidazole effects on microbiota and mucus layer thickness in the rat gut. FEMS Microbiol Ecol. 2010; 73:601–610.
Article52. van der Waaij LA, Harmsen HJ, Madjipour M, Kroese FG, Zwiers M, van Dullemen HM, et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis. 2005; 11:865–871.
Article53. Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011; 4:603–611.
Article54. van der Meulen TA, Harmsen H, Bootsma H, Spijkervet F, Kroese F, Vissink A. The microbiome-systemic diseases connection. Oral Dis. 2016; 22:719–734.
Article55. Mogna L, Del Piano M, Deidda F, Nicola S, Soattini L, Debiaggi R, et al. Assessment of the in vitro inhibitory activity of specific probiotic bacteria against different Escherichia coli strains. J Clin Gastroenterol. 2012; 46:S29–S32.
Article56. Aloisio I, Santini C, Biavati B, Dinelli G, Cencič A, Chingwaru W, et al. Characterization of Bifidobacterium spp. strains for the treatment of enteric disorders in newborns. Appl Microbiol Biotechnol. 2012; 96:1561–1576.
Article57. Drago L, De Vecchi E, Gabrieli A, De Grandi R, Toscano M. Immunomodulatory effects of Lactobacillus salivarius LS01 and Bifidobacterium breve BR03, alone and in combination, on peripheral blood mononuclear cells of allergic asthmatics. Allergy Asthma Immunol Res. 2015; 7:409–413.
Article58. Klemenak M, Dolinšek J, Langerholc T, Di Gioia D, Mičetić-Turk D. Administration of Bifidobacterium breve decreases the production of TNF-α in children with celiac disease. Dig Dis Sci. 2015; 60:3386–3392.
Article59. Ozkanli S, Karadag AS, Ozlu E, Uzuncakmak TK, Takci Z, Zemheri E, et al. A comparative study of MMP-1, MMP-2, and TNF-α expression in different acne vulgaris lesions. Int J Dermatol. 2016; 55:1402–1407.
Article60. Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013; 17:114–122.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Urticaria after Minocycline Therapy for Acne
- Gender-Specific Differences in Gut Microbiota Composition Associated with Microbial Metabolites for Patients with Acne Vulgaris
- Exfoliative Dermatitis Induced by Minocycline
- Four Cases of Minocycline-Induced Hyperpigmentation of the Tongue
- Host-microbial Cross-talk in Inflammatory Bowel Disease