Korean J Radiol.
2020 Jan;21(1):77-87. 10.3348/kjr.2019.0406.
Feasibility of Simultaneous Multislice Acceleration Technique in Diffusion-Weighted Magnetic Resonance Imaging of the Rectum
- Affiliations
-
- 1Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. sldmsdl@yuhs.ac
- 2Siemens Healthineers Korea, Seoul, Korea.
- KMID: 2467044
- DOI: http://doi.org/10.3348/kjr.2019.0406
Abstract
OBJECTIVE
To assess the feasibility of simultaneous multislice-accelerated diffusion-weighted imaging (SMS-DWI) of the rectum in comparison with conventional DWI (C-DWI) in rectal cancer patients.
MATERIALS AND METHODS
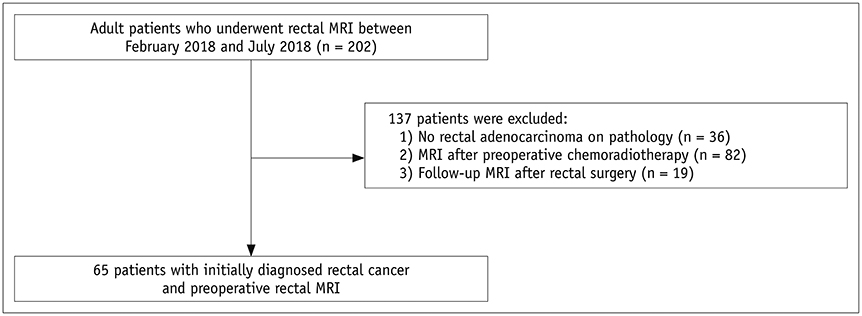
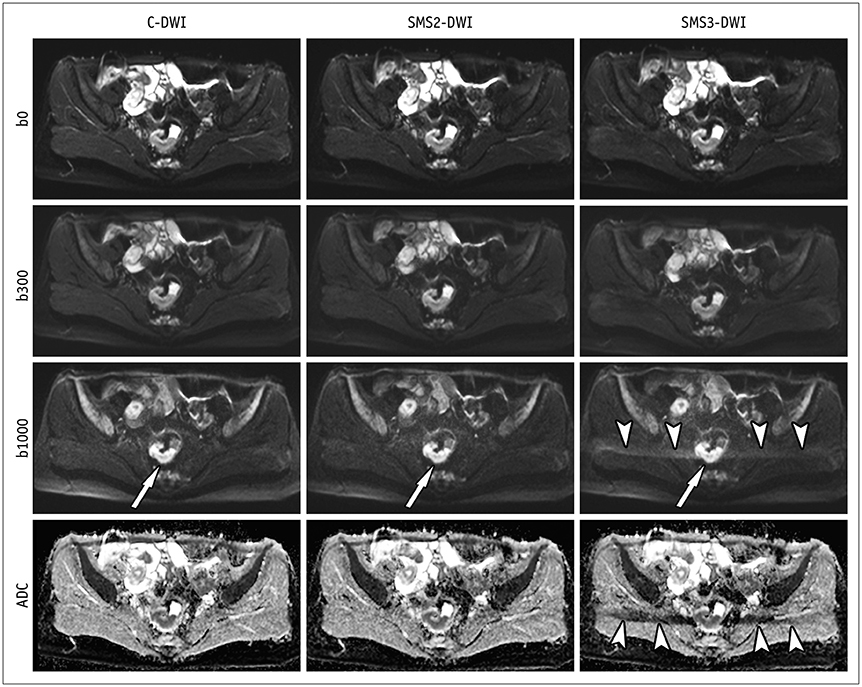
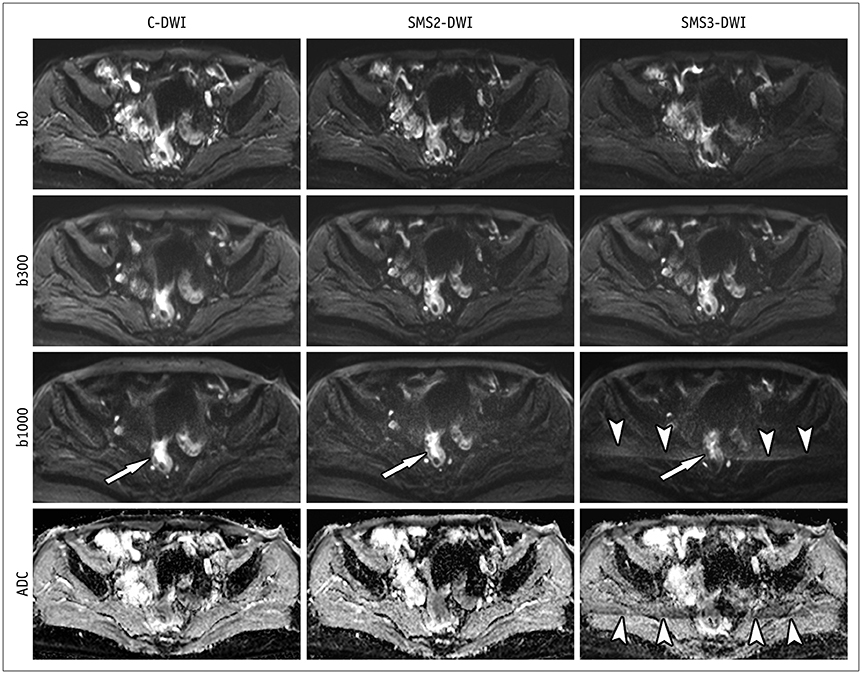
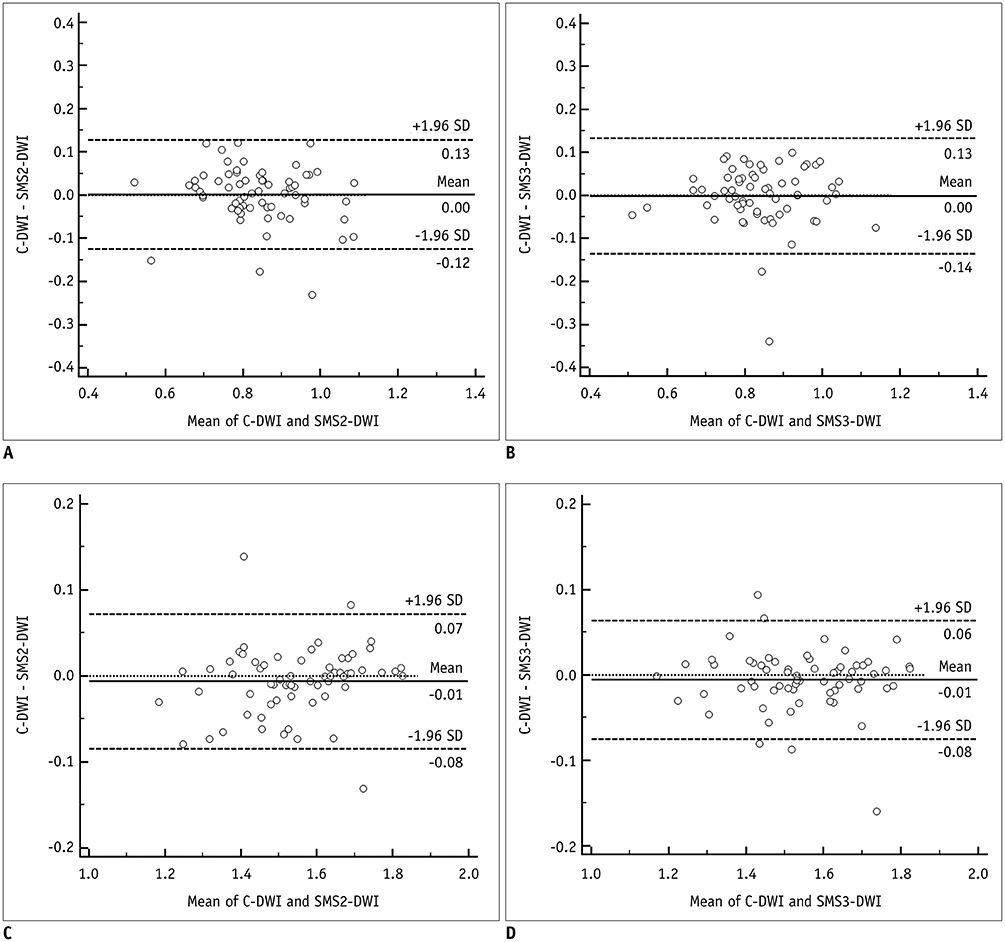
This study included 65 patients with initially-diagnosed rectal cancer. All patients underwent C-DWI and SMS-DWI with acceleration factors of 2 and 3 (SMS2-DWI and SMS3-DWI, respectively) using a 3T scanner. Acquisition times of the three DWI sequences were measured. Image quality in the three DWI sequences was reviewed by two independent radiologists using a 4-point Likert scale and subsequently compared using the Friedman test. Apparent diffusion coefficient (ADC) values for rectal cancer and the normal rectal wall were compared among the three sequences using repeated measures analysis of variance.
RESULTS
Acquisition times using C-DWI, SMS2-DWI, and SMS3-DWI were 173 seconds, 107 seconds, (38.2% shorter than C-DWI), and 77 seconds (55.5% shorter than C-DWI), respectively. For all image quality parameters other than distortion (margin sharpness, artifact, lesion conspicuity, and overall image quality), C-DWI and SMS2-DWI yielded better results than did SMS3-DWI (Ps < 0.001), with no significant differences observed between C-DWI and SMS2-DWI (Ps ≥ 0.054). ADC values of rectal cancer (p = 0.943) and normal rectal wall (p = 0.360) were not significantly different among C-DWI, SMS2-DWI, and SMS3-DWI.
CONCLUSION
SMS-DWI using an acceleration factor of 2 is feasible for rectal MRI resulting in substantial reductions in acquisition time while maintaining diagnostic image quality and similar ADC values to those of C-DWI.
Figure
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.
Article2. Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2012; 32:389–409.
Article3. KSAR Study Group for Rectal Cancer. Essential items for structured reporting of rectal cancer MRI: 2016 consensus recommendation from the Korean Society of Abdominal Radiology. Korean J Radiol. 2017; 18:132–151.4. Liu L, Liu Y, Xu L, Li Z, Lv H, Dong N, et al. Application of texture analysis based on apparent diffusion coefficient maps in discriminating different stages of rectal cancer. J Magn Reson Imaging. 2017; 45:1798–1808.
Article5. Lu ZH, Hu CH, Qian WX, Cao WH. Preoperative diffusion-weighted imaging value of rectal cancer: preoperative T staging and correlations with histological T stage. Clin Imaging. 2016; 40:563–568.
Article6. Choi MH, Oh SN, Rha SE, Choi JI, Lee SH, Jang HS, et al. Diffusion-weighted imaging: apparent diffusion coefficient histogram analysis for detecting pathologic complete response to chemoradiotherapy in locally advanced rectal cancer. J Magn Reson Imaging. 2016; 44:212–220.
Article7. Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011; 260:734–743.
Article8. Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology. 2011; 260:771–780.
Article9. Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018; 28:1465–1475.
Article10. Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010; 5:e15710.11. Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, et al. Evaluation of slice accelerations using multiband echo planar imaging at 3 T. Neuroimage. 2013; 83:991–1001.12. Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010; 63:1144–1153.
Article13. Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, et al. WU-Minn HCP Consortium. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012; 62:2222–2223.
Article14. Taron J, Martirosian P, Erb M, Kuestner T, Schwenzer NF, Schmidt H, et al. Simultaneous multislice diffusion-weighted MRI of the liver: analysis of different breathing schemes in comparison to standard sequences. J Magn Reson Imaging. 2016; 44:865–879.
Article15. Kenkel D, Barth BK, Piccirelli M, Filli L, Finkenstädt T, Reiner CS, et al. Simultaneous multislice diffusion-weighted imaging of the kidney: a systematic analysis of image quality. Invest Radiol. 2017; 52:163–169.16. Weiss J, Martirosian P, Taron J, Othman AE, Kuestner T, Erb M, et al. Feasibility of accelerated simultaneous multislice diffusion-weighted MRI of the prostate. J Magn Reson Imaging. 2017; 46:1507–1515.
Article17. Filli L, Piccirelli M, Kenkel D, Guggenberger R, Andreisek G, Beck T, et al. Simultaneous multislice echo planar imaging with blipped controlled aliasing in parallel imaging results in higher acceleration: a promising technique for accelerated diffusion tensor imaging of skeletal muscle. Invest Radiol. 2015; 50:456–463.18. Lau AZ, Tunnicliffe EM, Frost R, Koopmans PJ, Tyler DJ, Robson MD. Accelerated human cardiac diffusion tensor imaging using simultaneous multislice imaging. Magn Reson Med. 2015; 73:995–1004.
Article19. Ciritsis A, Rossi C, Marcon M, Van VDP, Boss A. Accelerated diffusion-weighted imaging for lymph node assessment in the pelvis applying simultaneous multislice acquisition: a healthy volunteer study. Medicine (Baltimore). 2018; 97:e1174.20. Dyde R, Chapman AH, Gale R, Mackintosh A, Tolan DJ. Precautions to be taken by radiologists and radiographers when prescribing hyoscine-N-butylbromide. Clin Radiol. 2008; 63:739–743.
Article21. Taron J, Martirosian P, Kuestner T, Schwenzer NF, Othman A, Weiß J, et al. Scan time reduction in diffusion-weighted imaging of the pancreas using a simultaneous multislice technique with different acceleration factors: how fast can we go? Eur Radiol. 2018; 28:1504–1511.
Article22. Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques. Magn Reson Med. 2016; 75:63–81.
Article23. Gagoski BA, Bilgic B, Eichner C, Bhat H, Grant PE, Wald LL, et al. RARE/turbo spin echo imaging with simultaneous multislice wave-CAIPI. Magn Reson Med. 2015; 73:929–938.
Article24. Obele CC, Glielmi C, Ream J, Doshi A, Campbell N, Zhang HC, et al. Simultaneous multislice accelerated free-breathing diffusion-weighted imaging of the liver at 3T. Abdom Imaging. 2015; 40:2323–2330.
Article25. Boss A, Barth B, Filli L, Kenkel D, Wurnig MC, Piccirelli M, et al. Simultaneous multi-slice echo planar diffusion weighted imaging of the liver and the pancreas: optimization of signal-to-noise ratio and acquisition time and application to intravoxel incoherent motion analysis. Eur J Radiol. 2016; 85:1948–1955.
Article26. Taron J, Weiß J, Martirosian P, Seith F, Stemmer A, Bamberg F, et al. Clinical robustness of accelerated and optimized abdominal diffusion-weighted imaging. Invest Radiol. 2017; 52:590–595.
Article27. Peng Y, Li Z, Tang H, Wang Y, Hu X, Shen Y, et al. Comparison of reduced field-of-view diffusion-weighted imaging (DWI) and conventional DWI techniques in the assessment of rectal carcinoma at 3.0T: image quality and histological T staging. J Magn Reson Imaging. 2018; 47:967–997.
Article28. Akashi M, Nakahusa Y, Yakabe T, Egashira Y, Koga Y, Sumi K, et al. Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta Radiol. 2014; 55:524–531.
Article29. Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007; 26:375–385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RE: Diffusion-Weighted Imaging of Prostate Cancer: How Can We Use It Accurately?
- Diffusion-Weighted Magnetic Resonance Imaging of Spine
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Diffusion-Weighted Magnetic Resonance Imaging Findings in a Patient with Trigeminal Ganglioneuroma
- The Value of PROPELLER Diffusion-Weighted Image in the Detection of Cholesteatoma