J Nutr Health.
2019 Feb;52(1):17-25. 10.4163/jnh.2019.52.1.17.
Effects of Lonicera caerulea extract on adipocyte differentiation and adipogenesis in 3T3-L1 cells and mouse adipose-derived stem cells (MADSCs)
- Affiliations
-
- 1Department of Food and Nutrition, Gachon University, Seongnam, Gyeonggi 13120, Korea. skysea@gachon.ac.kr
- 2Research Group of Functional Food Materials, Korea Food Research Institute, Wanju, Jeonbuk 55365, Korea.
- KMID: 2466679
- DOI: http://doi.org/10.4163/jnh.2019.52.1.17
Abstract
- PURPOSE
Obesity is a major health problem of global significance because it is clearly associated with an increased risk of health problems, such as nonalcoholic fatty liver disease (NAFLD), diabetes, cardiovascular diseases, and cancer. Lonicera caerulea (LC) originates from high mountains or wet areas and has been used as a traditional medicine in northern Russia, China, and Japan. LC contains a range of bioactive constituents, such as vitamins, minerals, and polyphenols. This study examined the anti-obesity effects of LC during differentiation in preadipocytes.
METHODS
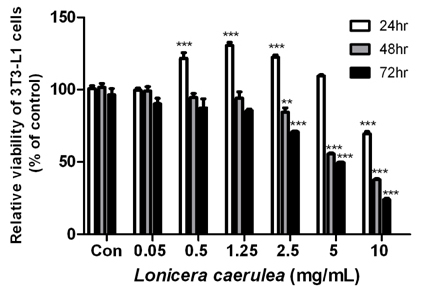
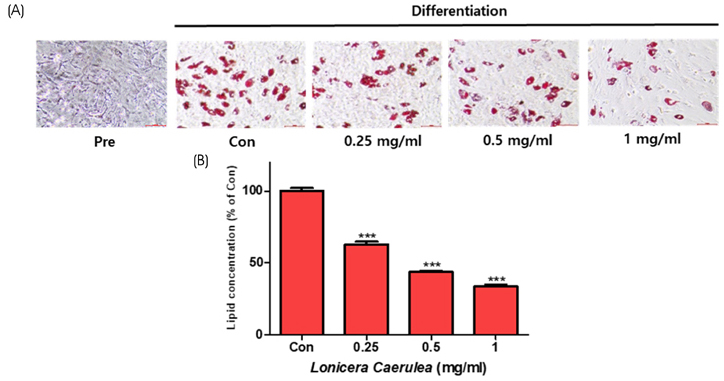
The cell viability assay was performed after the differentiation of 3T3-L1 cells for 7 days. Oil Red O staining was used to visualize the changes in lipid droplets in 3T3-L1 cells and mouse adipose-derived stem cells (MADSCs). The mRNA expression of obesity-related genes was determined by quantitative real-time PCR.
RESULTS
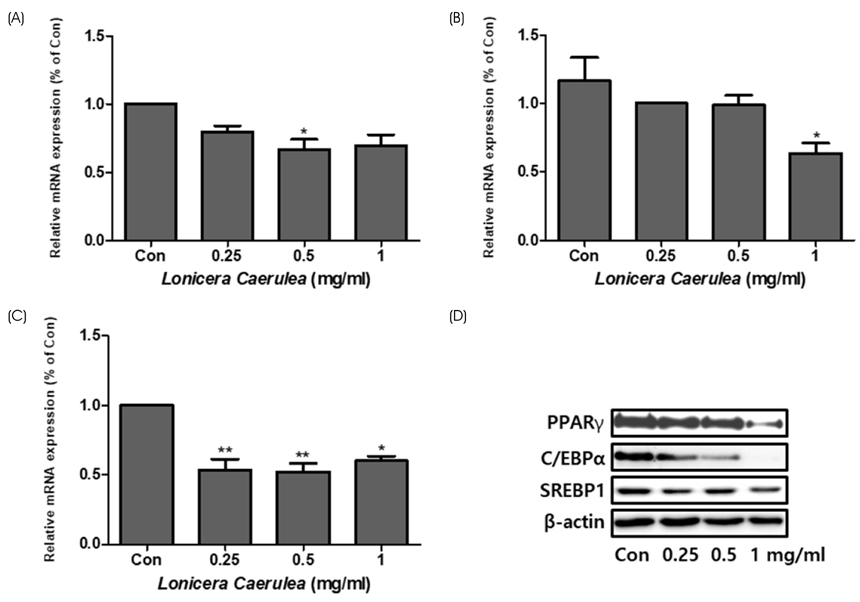
According to the results of Oil Red O staining, the lipid levels and size of lipid droplets in the adipocytes were reduced and the LC extract (LCE, 0.25-1 mg/mL) markedly inhibited adipogenesis in a dose-dependent manner. The treatment of LCE also decreased the mRNA expression of peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein-α (C/EBPα), and sterol regulatory element binding protein 1 (SREBP1) in 3T3-L1 cells. Western blot analysis showed that the PPARγ, C/EBPα, and SREBP1 protein levels in both 3T3-L1 and MADSC were reduced in a dose-dependent manner.
CONCLUSION
These results suggest that LCE can inhibit adipogenic differentiation through the regulation of adipogenesis-related markers.
MeSH Terms
-
3T3-L1 Cells*
Adipocytes*
Adipogenesis*
Animals
Blotting, Western
Cardiovascular Diseases
Cell Survival
China
Japan
Lipid Droplets
Lonicera*
Medicine, Traditional
Mice*
Minerals
Miners
Non-alcoholic Fatty Liver Disease
Obesity
Peroxisomes
Polyphenols
Real-Time Polymerase Chain Reaction
RNA, Messenger
Russia
Stem Cells*
Sterol Regulatory Element Binding Protein 1
Vitamins
Minerals
Polyphenols
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Vitamins
Figure
Reference
-
1. Yatsuya H, Li Y, Hilawe EH, Ota A, Wang C, Chiang C, et al. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ J. 2014; 78(12):2807–2818.
Article2. Kopelman PG. Obesity as a medical problem. Nature. 2000; 404(6778):635–643.
Article3. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and pathway. Toxicol Res. 2018; 34(1):13–21.4. Bell L, Lamport DJ, Butler LT, Williams CM. A review of the cognitive effects observed in humans following acute supplementation with flavonoids, and their associated mechanisms of action. Nutrients. 2015; 7(12):10290–10306.
Article5. Han MH, Kim HJ, Jeong JW, Park C, Kim BW, Choi YH. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of Vitis coignetiae pulliat is associated with the activation of AMPK signaling pathway. Toxicol Res. 2018; 34(1):13–21.6. Leu SY, Chen YC, Tsai YC, Hung YW, Hsu CH, Lee YM, et al. Raspberry ketone reduced lipid accumulation in 3T3-L1 cells and ovariectomy-induced obesity in wistar rats by regulating autophagy mechanisms. J Agric Food Chem. 2017; 65(50):10907–10914.
Article7. Rupasinghe HP, Yu LJ, Bhullar KS, Bors B. Short communication: haskap (Lonicera caerulea): a new berry crop with high antioxidant capacity. Can J Plant Sci. 2012; 92(7):1311–1317.
Article8. Kähkönen MP, Hopia AI, Heinonen M. Berry phenolics and their antioxidant activity. J Agric Food Chem. 2001; 49(8):4076–4082.
Article9. Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000; 48(2):140–146.
Article10. Han SJ. Protective efficacies of Aronia melanocarpa (blackberry) on the allyl alcohol-damaged hepatocyte of mice. Korean J Pharmacogn. 2013; 44(1):91–96.11. Kim JH, Jo MN, Han JH, Pyo SH, Kim TS, Kim EH, et al. Anti-inflammatory activity of the fruits of blue honeysuckle. Yakhak Hoeji. 2016; 60(5):235–242.
Article12. Ko GA, Koh YS, Ryu JY, Kim Cho S. Comparison of proximate compositions, antioxidant, and antiproliferative activities between blueberry and Sageretia thea (Osbeck) M.C. Johnst fruit produced in Jeju Island. J Appl Biol Chem. 2017; 60(2):161–171.13. Zhao H, Wang Z, Ma F, Yang X, Cheng C, Yao L. Protective effect of anthocyanin from Lonicera caerulea var. edulis on radiation-induced damage in mice. Int J Mol Sci. 2012; 13(9):11773–11782.
Article14. Vostálová J, Galandáková A, Palíková I, Ulrichová J, Doležal D, Lichnovská R, et al. Lonicera caerulea fruits reduce UVA-induced damage in hairless mice. J Photochem Photobiol B. 2013; 128:1–11.
Article15. Aune UL, Ruiz L, Kajimura S. Isolation and differentiation of stromal vascular cells to beige/brite cells. J Vis Exp. 2013; (73):50191.
Article16. Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005; 54(3):132–141.
Article17. Harmon AW, Harp JB. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol. 2001; 280(4):C807–C813.
Article18. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006; 7(12):885–896.
Article19. Lee YM, Yoon Y, Yoon H, Park HM, Song S, Yeum KJ. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017; 9(10):pii: E1089.
Article20. Azzini E, Giacometti J, Russo GL. Antiobesity effects of anthocyanins in preclinical and clinical studies. Oxid Med Cell Longev. 2017; 2017:2740364.
Article21. Lee B, Lee M, Lefevre M, Kim HR. Anthocyanins inhibit lipogenesis during adipocyte differentiation of 3T3-L1 preadipocytes. Plant Foods Hum Nutr. 2014; 69(2):137–141.
Article22. Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr Opin Genet Dev. 1995; 5(5):571–576.
Article23. Wu Z, Puigserver P, Spiegelman BM. Transcriptional activation of adipogenesis. Curr Opin Cell Biol. 1999; 11(6):689–694.
Article24. Oh SW. Recent epidemiological changes in Korean obesity. Korean J Helicobacter Up Gastrointest Res. 2017; 17(2):62–65.
Article25. Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring). 2016; 24(3):597–605.
Article26. Ailhaud G, Grimaldi P, Négrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992; 12(1):207–233.
Article27. MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1995; 92(20):9034–9037.
Article28. Yu S, Shin S. Effects of Carthamus tinctorius extract on adipogenic differentiation of mouse bone marrow-derived mesenchymal stromal stem cells. J Int Korean Med. 2017; 38(1):1–9.
Article29. Chen G, Li H, Zhao Y, Zhu H, Cai E, Gao Y, et al. Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice. Food Chem Toxicol. 2017; 106(Pt A):393–403.
Article30. Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010; 21(11):1783–1787.
Article31. Grégoire F, Todoroff G, Hauser N, Remacle C. The stroma-vascular fraction of rat inguinal and epididymal adipose tissue and the adipoconversion of fat cell precursors in primary culture. Biol Cell. 1990; 69(3):215–222.32. Pandurangan M, Park J, Kim E. Aspartame downregulates 3T3-L1 differentiation. In Vitro Cell Dev Biol Anim. 2014; 50(9):851–857.
Article33. Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991; 251(4991):288–292.
Article34. Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994; 14(1):99–129.
Article35. Smolik M, Ochmian I, Grajkowski J. Genetic variability of Polish and Russian accessions of cultivated blue honeysuckle (Lonicera caerulea). Genetika. 2010; 46(8):1079–1085. DOI: 10.1134/s1022795410080077.
Article36. Plekhanova MN. Blue honeysuckle (Lonicera caerulea L.) - a new commercial berry crop for temperate climate: genetic resources and breeding. Acta Hortic. 2000; 538(538):159–164.
Article37. Skupień K, Ochmian I, Grajkowski J. Influence of ripening time on fruit chemical composition of two blue honeysuckle cultigens. J Fruit Ornam Plant Res. 2009; 17(1):101–111.38. Joseph SV, Edirisinghe I, Burton-Freeman BM. Berries: anti-inflammatory effects in humans. J Agric Food Chem. 2014; 62(18):3886–3903.
Article39. Song Y, Park HJ, Kang SN, Jang SH, Lee SJ, Ko YG, et al. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS One. 2013; 8(7):e69925.
Article40. Tsuda T. Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants (Basel). 2016; 5(2):pii: E13.
Article41. Kim HK, Kim JN, Han SN, Nam JH, Na HN, Ha TJ. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr Res. 2012; 32(10):770–777.
Article42. Khan MI, Shin JH, Shin TS, Kim MY, Cho NJ, Kim JD. Anthocyanins from Cornus kousa ethanolic extract attenuate obesity in association with anti-angiogenic activities in 3T3-L1 cells by down-regulating adipogeneses and lipogenesis. PLoS One. 2018; 13(12):e0208556.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Funding Acknowledgment Correction. Effects of Lonicera caerulea extract on adipocyte differentiation and adipogenesis in 3T3-L1 cells and mouse adipose-derived stem cells (MADSCs)
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- TonEBP suppresses adipocyte differentiation via modulation of early signaling in 3T3-L1 cells
- Expression of Growth Hormone Receptor Gene During 3T3-L1 Differentiation