Neonatal Med.
2019 Nov;26(4):191-197. 10.5385/nm.2019.26.4.191.
Inhaled Iloprost as a First-Line Therapy for Persistent Pulmonary Hypertension of the Newborn
- Affiliations
-
- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea. neopark@hanyang.ac.kr
- KMID: 2466638
- DOI: http://doi.org/10.5385/nm.2019.26.4.191
Abstract
- PURPOSE
Persistent pulmonary hypertension of the newborn (PPHN) is a potentially fatal disease. Inhaled iloprost, a stable analogue of prostacyclin, has recently been used as a therapeutic option. However, there are no clinical guidelines on the use of iloprost, specifically for neonates. This study aimed to suggest the use of inhaled iloprost as a rescue therapy for PPHN based on our experience.
METHODS
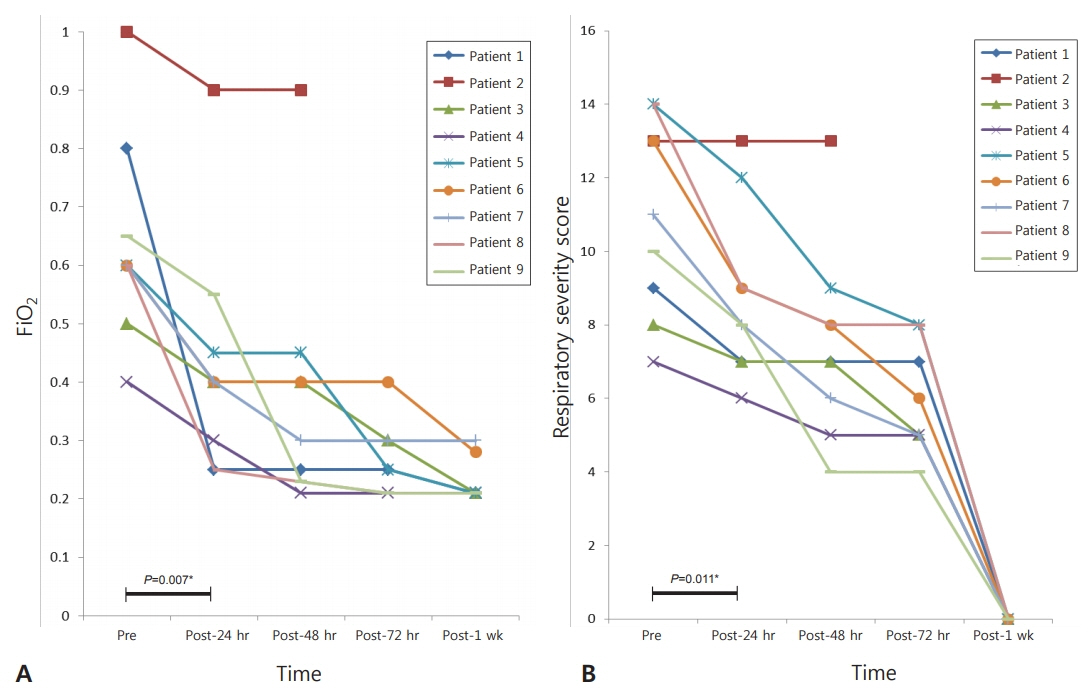
The efficacy and adverse events of inhaled iloprost were evaluated prospectively in nine full-term neonates with PPHN. We monitored the following parameters: fraction of inspired oxygen (FiOâ‚‚), respiratory severity score (RSS), heart rate, and mean blood pressure.
RESULTS
The inhalation dose was 1 to 2 µg/kg initially, and 4 to 8 inhalations per day were applied over 2 to 8 days, except in the case of one neonate who died 2 days after birth. Echocardiographic findings, changes in FiOâ‚‚, and RSS improved within the next 7 days in eight of the nine patients. Severe side effects on heart rate and blood pressure were not observed.
CONCLUSION
Our experience suggests that inhaled iloprost can be used as a first-line treatment in newborn infants with PPHN when inhaled nitric oxide is not available. To the best of our knowledge, this report is the first prospective case series on the use of inhaled iloprost in PPHN.
MeSH Terms
Figure
Reference
-
1. Nair J, Lakshminrusimha S. Update on PPHN: mechanisms and treatment. Semin Perinatol. 2014; 38:78–91.2. Maxey DM, Ivy DD, Ogawa MT, Feinstein JA. Food and Drug Administration (FDA) postmarket reported side effects and adverse events associated with pulmonary hypertension therapy in pediatric patients. Pediatr Cardiol. 2013; 34:1628–36.3. Ehlen M, Wiebe B. Iloprost in persistent pulmonary hypertension of the newborn. Cardiol Young. 2003; 13:361–3.4. Avila-Alvarez A, Bravo-Laguna MC, Bronte LD, Del Cerro MJ. Inhaled iloprost as a rescue therapy for transposition of the great arteries with persistent pulmonary hypertension of the newborn. Pediatr Cardiol. 2013; 34:2027–9.5. De Luca D, Zecca E, Piastra M, Romagnoli C. Iloprost as ‘rescue’ therapy for pulmonary hypertension of the neonate. Paediatr Anaesth. 2007; 17:394–5.6. Yilmaz O, Kahveci H, Zeybek C, Ciftel M, Kilic O. Inhaled iloprost in preterm infants with severe respiratory distress syndrome and pulmonary hypertension. Am J Perinatol. 2014; 31:321–6.7. Chotigeat U, Jaratwashirakul S. Inhaled iloprost for severe persistent pulmonary hypertension of the newborn. J Med Assoc Thai. 2007; 90:167–70.8. Limsuwan A, Wanitkul S, Khosithset A, Attanavanich S, Samankatiwat P. Aerosolized iloprost for postoperative pulmonary hypertensive crisis in children with congenital heart disease. Int J Cardiol. 2008; 129:333–8.9. Shah PS, Ohlsson A. Sildenafil for pulmonary hypertension in neonates. Cochrane Database Syst Rev. 2007; 3:CD005494.10. Dhillon R. The management of neonatal pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 2012; 97:F223–8.11. Kirbas A, Yalcin Y, Tanrikulu N, Gurer O, Isik O. Comparison of inhaled nitric oxide and aerosolized iloprost in pulmonary hypertension in children with congenital heart surgery. Cardiol J. 2012; 19:387–94.12. Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007; 131:1917–28.13. Piastra M, De Luca D, De Carolis MP, Tempera A, Stival E, Caliandro F, et al. Nebulized iloprost and noninvasive respiratory support for impending hypoxaemic respiratory failure in formerly preterm infants: a case series. Pediatr Pulmonol. 2012; 47:757–62.14. Eifinger F, Sreeram N, Mehler K, Huenseler C, Kribs A, Roth B. Aerosolized iloprost in the treatment of pulmonary hypertension in extremely preterm infants: a pilot study. Klin Padiatr. 2008; 220:66–9.15. Hwang SK, O YC, Kim NS, Park HK, Yum MK. Use of inhaled iloprost in an infant with bronchopulmonary dysplasia and pulmonary artery hypertension. Korean Circ J. 2009; 39:343–5.16. Gurakan B, Kayiran P, Ozturk N, Kayiran SM, Dindar A. Therapeutic combination of sildenafil and iloprost in a preterm neonate with pulmonary hypertension. Pediatr Pulmonol. 2011; 46:617–20.17. Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2012; 32:608–13.18. Kahveci H, Yilmaz O, Avsar UZ, Ciftel M, Kilic O, Laloglu F, et al. Oral sildenafil and inhaled iloprost in the treatment of pulmonary hypertension of the newborn. Pediatr Pulmonol. 2014; 49:1205–13.19. Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. 2012; 64:540–82.20. Han YK, Lee SM, Eun HS, Kim JE, Namgung R, Park MS, et al. The therapeutic effect of inhaled iloprost in newborn infants with severe persistent pulmonary hypertension refractory to inhaled nitric oxide. Korean J Perinatol. 2011; 22:57–63.21. Mulligan C, Beghetti M. Inhaled iloprost for the control of acute pulmonary hypertension in children: a systematic review. Pediatr Crit Care Med. 2012; 13:472–80.22. Eronen M, Pohjavuori M, Andersson S, Pesonen E, Raivio KO. Prostacyclin treatment for persistent pulmonary hypertension of the newborn. Pediatr Cardiol. 1997; 18:3–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of persistent pulmonary hypertension of the newborn: Treatment with inhaled iloprost
- The Therapeutic Effect of Inhaled Iloprost in Newborn Infants with Severe Persistent Pulmonary Hypertension Refractory to Inhaled Nitric Oxide
- Pulmonary Arterial Hypertension is Normalized Following Six Years of Inhaled Iloprost Treatment in a Patient with Systemic Sclerosis
- Use of Inhaled Iloprost in an Infant With Bronchopulmonary Dysplasia and Pulmonary Artery Hypertension
- The effect of perioperative inhaled iloprost on congenital heart disease with severe pulmonary arterial hypertension