Korean J Physiol Pharmacol.
2020 Jan;24(1):47-52. 10.4196/kjpp.2020.24.1.47.
Bordetella bronchiseptica is a potent and safe adjuvant that enhances the antigen-presenting capability of dendritic cells
- Affiliations
-
- 1College of Veterinary Medicine, Jeju National University, Jeju 63243, Korea. jooh@jejunu.ac.kr
- 2Veterinary Medical Research Institute, Jeju National University, Jeju 63243, Korea.
- KMID: 2466569
- DOI: http://doi.org/10.4196/kjpp.2020.24.1.47
Abstract
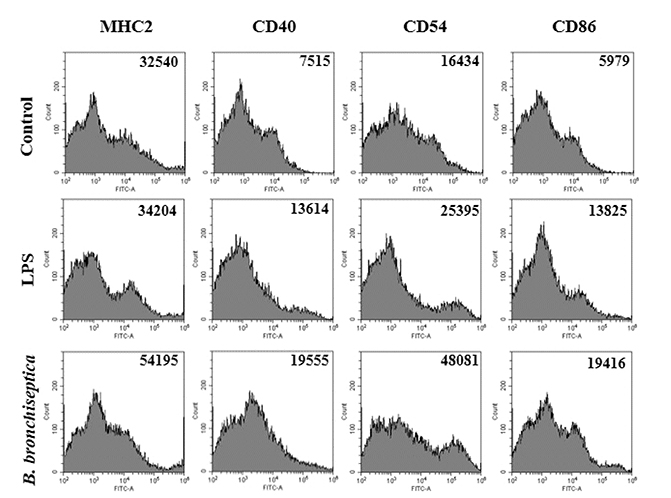
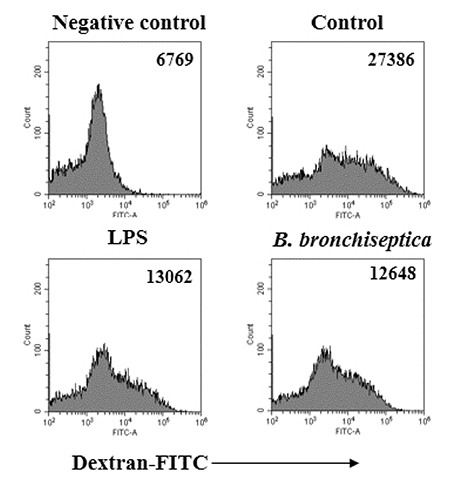
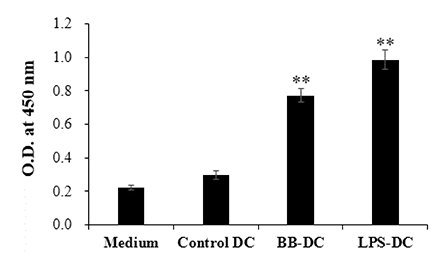
- We previously demonstrated that Bordetella bronchiseptica (B. bronchiseptica) antigen (Ag) enhances the Mycoplasma hyopneumoniae Ag-specific immune response. The focus of this study was whether acellular bacterin of B. bronchiseptica could be used as an adjuvant to increase antigen-presenting capability of dendritic cells (DCs) by increasing the level of activation. The metabolic activity of DCs was increased by B. bronchiseptica, similar to lipopolysaccharide (LPS). Flow cytometry analysis revealed that B. bronchiseptica increases the expression of major histocompatibility complex class-2, cluster of differentiation (CD)40, CD54, and CD86 which are closely related to DC-mediated immune responses. B. bronchiseptica enhanced the production of cytokines related to adaptive immune responses. Furthermore, the survival rate of B. bronchiseptica-injected groups was 100% at 15 and 20 mg/kg doses, whereas that of LPS-injected groups was only 20%, 0% at 15 and 20 mg/kg doses respectively, and so B. bronchiseptica is likely to be safer than LPS. Taken together, these results indicate that B. bronchiseptica can be used as an adjuvant to enhance the antigen-presenting capability of DCs. B. bronchiseptica is a candidate for producing vaccines, especially in case of DC-mediating efficacy and safety demands. This study provides researchers and clinicians with valuable information regarding the usage of B. bronchiseptica as a safe bacteria-derived immunostimulating agent for developing efficient vaccines.
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of the effects of disulfiram, an alcohol-aversive agent with anti-cancer activity, on mouse bone marrow cells
Seo-Ro Park, Hong-Gu Joo
Korean J Physiol Pharmacol. 2022;26(3):157-164. doi: 10.4196/kjpp.2022.26.3.157.
Reference
-
1. Karch CP, Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol. 2016; 120:1–14.
Article2. Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003; 8:934–943.
Article3. Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: a review on adjuvants in licensed vaccines. Semin Immunol. 2018; 39:14–21.
Article4. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010; 33:492–503.
Article5. Dunne A, Mielke LA, Allen AC, Sutton CE, Higgs R, Cunningham CC, Higgins SC, Mills KH. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 2015; 8:607–617.
Article6. Wangoo A, Brown IN, Marshall BG, Cook HT, Young DB, Shaw RJ. Bacille Calmette-Guérin (BCG)-associated inflammation and fibrosis: modulation by recombinant BCG expressing interferon-gamma (IFN-gamma). Clin Exp Immunol. 2000; 119:92–98.7. Filardi MJ, Codish SD, Civerchia L, Howard RK, McKneally MF. Toxicity of intrapleural Bacillus Calmette-Guérin treatment in animals. Cancer Res. 1979; 39:3673–3676.8. Gordon S, Keshav S, Stein M. BCG-induced granuloma formation in murine tissues. Immunobiology. 1994; 191:369–377.
Article9. Fedele G, Celestino I, Spensieri F, Frasca L, Nasso M, Watanabe M, Remoli ME, Coccia EM, Altieri F, Ausiello CM. Lipooligosaccharide from Bordetella pertussis induces mature human monocyte-derived dendritic cells and drives a Th2 biased response. Microbes Infect. 2007; 9:855–863.
Article10. Samore MH, Siber GR. Pertussis toxin enhanced IgG1 and IgE responses to primary tetanus immunization are mediated by interleukin-4 and persist during secondary responses to tetanus alone. Vaccine. 1996; 14:290–297.
Article11. Zhao Z, Wang C, Xue Y, Tang X, Wu B, Cheng X, He Q, Chen H. The occurrence of Bordetella bronchiseptica in pigs with clinical respiratory disease. Vet J. 2011; 188:337–340.
Article12. Goodnow RA. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980; 44:722–738.
Article13. Chanter N, Magyar T, Rutter JM. Interactions between Bordetella bronchiseptica and toxigenic Pasteurella multocida in atrophic rhinitis of pigs. Res Vet Sci. 1989; 47:48–53.
Article14. Horiguchi Y. Swine atrophic rhinitis caused by pasteurella multocida toxin and bordetella dermonecrotic toxin. Curr Top Microbiol Immunol. 2012; 361:113–129.
Article15. Siciliano NA, Skinner JA, Yuk MH. Bordetella bronchiseptica modulates macrophage phenotype leading to the inhibition of CD4+ T cell proliferation and the initiation of a Th17 immune response. J Immunol. 2006; 177:7131–7138.16. Yim SH, Hahn TW, Joo HG. Bordetella bronchiseptica antigen enhances the production of Mycoplasma hyopneumoniae antigen-specific immunoglobulin G in mice. J Vet Sci. 2017; 18:327–332.17. Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999; 5:1249–1255.
Article18. Théry C, Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol. 2001; 13:45–51.
Article19. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991; 174:1209–1220.
Article20. Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991; 147:3815–3822.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stimulatory effects of Bordetella bronchiseptica antigen on bone marrow cells and immune memory responses
- Evaluation of the immunogenicity of Bordetella bronchiseptica, a vaccine antigen
- Bordetella bronchiseptica antigen enhances the production of Mycoplasma hyopneumoniae antigen-specific immunoglobulin G in mice
- Bordetella bronchiseptica Respiratory Infection in the Immunosuppressed Patient
- Evaluation of adjuvant effects of fucoidan for improving vaccine efficacy