Yonsei Med J.
2020 Jan;61(1):79-84. 10.3349/ymj.2020.61.1.79.
Randomized, Double-Blind, Placebo-Controlled Trial on the Efficacy of Hyaluronidase in Preventing Perineal Trauma in Nulliparous Women
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
- 2Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Kangbuk Samsung Hospital, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- 5Department of Obstetrics and Gynecology, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, Korea.
- 6Department of Obstetrics and Gynecology, Soonchunhyang University Seoul Hospital, Seoul, Korea. kychoi@schmc.ac.kr
- KMID: 2466339
- DOI: http://doi.org/10.3349/ymj.2020.61.1.79
Abstract
- PURPOSE
Hyaluronidase (HAase) has many uses in medicine, and reports suggest that it affects perineal tissue during fetal passage through the vaginal canal. However, its potential use for preventing perineal trauma has yet to be determined. This study sought to evaluate the efficacy and safety of perineal HAase injections in reducing perineal trauma during vaginal delivery.
MATERIALS AND METHODS
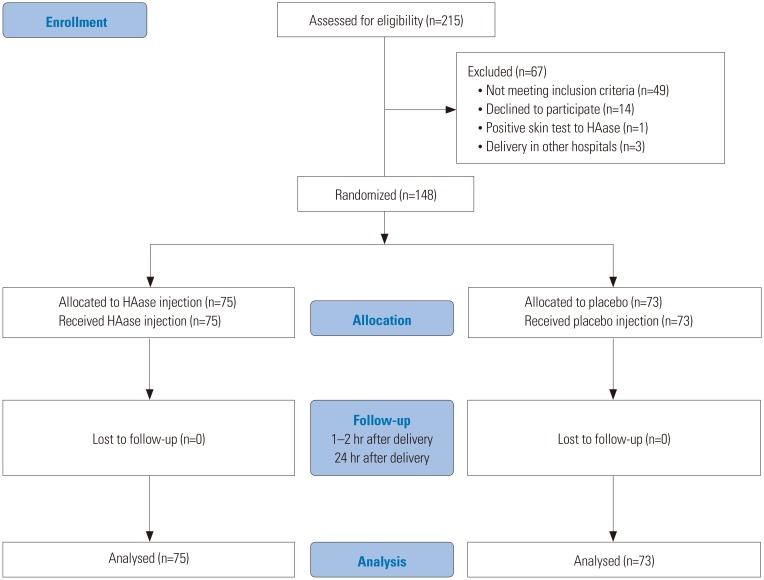
A multi-center, double-blind, placebo-controlled, randomized study was conducted from January 2016 to March 2017. Nulliparous women who planned to undergo vaginal delivery were recruited, and the enrolled women were randomly assigned to the HAase injection group (HAase injection, 5000 IU, n=75) or the control group (normal saline injection, n=73). The degree of perineal laceration, rate of episiotomy, and grade of perineal edema at 1 hour and 24 hours after spontaneous vaginal delivery were compared between the two groups.
RESULTS
A total of 148 women who underwent vaginal delivery were recruited. No significant differences were observed between the HAase injection and control groups in the rates of perineal laceration (p=0.422). Perineal edema significantly decreased 24 hours after delivery in the women treated with perineal HAase injections, compared to women in the control group (p=0.008). The overall incidences of adverse events, such as redness of the injection site, infection, and wound dehiscence, were similar between the two groups.
CONCLUSION
HAase injections in nulliparous women afforded no reductions in the rates of perineal lacerations and episiotomy. However, the use of perineal HAase injections did reduce perineal edema without severe adverse events.
MeSH Terms
Figure
Reference
-
1. Baumann P, Hammoud AO, McNeeley SG, DeRose E, Kudish B, Hendrix S. Factors associated with anal sphincter laceration in 40,923 primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:985–990. PMID: 17211527.
Article2. Fernando RJ, Sultan AH, Kettle C, Thakar R. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2013; (12):CD002866. PMID: 24318732.
Article3. Frohlich J, Kettle C. Perineal care. BMJ Clin Evid. 2015; 2015:1401.4. Marschalek ML, Worda C, Kuessel L, Koelbl H, Oberaigner W, Leitner H, et al. Risk and protective factors for obstetric anal sphincter injuries: a retrospective nationwide study. Birth. 2018; 45:409–415. PMID: 29537100.
Article5. Pauls RN, Silva WA, Rooney CM, Siddighi S, Kleeman SD, Dryfhout V, et al. Sexual function following anal sphincteroplasty for fecal incontinence. Am J Obstet Gynecol. 2007; 197:618. PMID: 18060952.
Article6. Bagade P, Mackenzie S. Outcomes from medium term follow-up of patients with third and fourth degree perineal tears. J Obstet Gynaecol. 2010; 30:609–612. PMID: 20701512.
Article7. Priddis H, Schmied V, Dahlen H. Women's experiences following severe perineal trauma: a qualitative study. BMC Womens Health. 2014; 14:32. PMID: 24559056.
Article8. Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998; 131:3–11. PMID: 9839614.
Article9. Lv SH, Rong SF, Cai BG, Guan SM, Li QQ. Property and current clinical applications of mammal hyaluronidase. Eur Rev Med Pharmacol Sci. 2015; 19:3968–3976. PMID: 26531287.10. Brown CR. Distribution of hyaluronidase in the ram spermatozoon. J Reprod Fertil. 1975; 45:537–539. PMID: 1243148.
Article11. Yuda Y, Kasashima Y, Kuwano A, Sato K, Hattori S, Arai K. Active hyaluronidase 2 expression in the granulation tissue formed in the healing process of equine superficial digital flexor tendonitis. J Vet Med Sci. 2013; 75:219–223. PMID: 23047331.
Article12. Narayanan R, Kuppermann BD. Hyaluronidase for pharmacologic vitreolysis. Dev Ophthalmol. 2009; 44:20–25. PMID: 19494648.
Article13. Wohlrab J, Finke R, Franke WG, Wohlrab A. Clinical trial for safety evaluation of hyaluronidase as diffusion enhancing adjuvant for infiltration analgesia of skin with lidocaine. Dermatol Surg. 2012; 38:91–96. PMID: 22093158.
Article14. Scarabotto LB, Riesco ML. Use of hyaluronidase to prevent perineal trauma during spontaneous delivery: a pilot study. J Midwifery Womens Health. 2008; 53:353–361. PMID: 18586189.
Article15. Zhou F, Wang XD, Li J, Huang GQ, Gao BX. Hyaluronidase for reducing perineal trauma. Cochrane Database Syst Rev. 2014; (2):CD010441. PMID: 24497276.
Article16. Digonnet L, Cahn J, Roy J, Boucet J. [Effect of local injection of hyaluronidase on perineal distention in labor in primiparas]. Therapie. 1952; 7:388–391. PMID: 13049228.17. Frenzel KH. Protection of the perineum with hyaluronidase. Zentralbl Gynakol. 1954; 76:1602–1604. PMID: 14349182.18. Mink E. [Experience with the softening effect of hyalurodase on high and rigid perineum in primiparas]. Geburtshilfe Frauenheilkd. 1955; 15:246–258. PMID: 14380684.19. Rimbach E, Griefahn S. Critical evaluation of perineum protection with hyaluronidase. Zentralbl Gynakol. 1955; 77:546–548. PMID: 14397711.20. Patrini G. [Usefulness of hyaluronidase in labor as protector of the perineum]. Riv Ostet Ginecol Prat. 1956; 38:710–714. PMID: 13380314.21. Petronio G. [Protective action of hyaluronidase on vaginoperineal lesions from spontaneous and surgical labor]. Minerva Med. 1956; 47:2071–2073. PMID: 13399919.22. Chatfield WR, Moir DD. The effect of hyaluronidase on the perineum. A controlled trial of 200 primigravid patients in labour. J Obstet Gynaecol Br Commonw. 1966; 73:670–671. PMID: 5912630.
Article23. Colacioppo PM, Gonzalez Riesco ML, Koiffman MD. Use of hyaluronidase to prevent perineal trauma during spontaneous births: a randomized, placebo-controlled, double-blind, clinical trial. J Midwifery Womens Health. 2011; 56:436–445. PMID: 23181640.
Article24. Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins;2007.25. Sultan AH, Fernando R. Maternal obstetric injury. Curr Obstet Gynaecol. 2001; 11:279–284.
Article26. Hogan MA, Madayag T. Medical-surgical nursing: reviews and rationales. 2nd ed. Salt Lake City (UT): Prentice Hall;2007.27. Kwon HY, Park HS. Episiotomy and the risk of severe perineal injuries among Korean women. J Matern Fetal Neonatal Med. 2017; 30:1745–1749. PMID: 27549862.
Article28. O'Leary JA, Erez S. Hyaluronidase as an adjuvant to episiotomy. Obstet Gynecol. 1965; 26:66–69.29. Senol DK, Aslan E. The effects of cold application to the perineum on pain relief after vaginal birth. Asian Nurs Res. 2017; 11:276–282.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adjuvant Sertraline Treallnent for Chronic Schizophrenia :A Randomized, Double Blind, Placeho-Controlled Study
- Effects of cetirizine in dogs with chronic atopic dermatitis: a randomized, double blind, placebo-controlled trial
- Efficacy and Safety of Low-Dose Gamma-Aminobutyric Acid From Unpolished Rice Germ as a Health Functional Food for Promoting Sleep: A Randomized, Double-Blind, Placebo-Controlled Trial
- Efficacy of Trimetazidine Dihydrochloride for Relieving Chronic Tinnitus: A Randomized Double-Blind Study
- Comparison of Vitex agnus-castus Extracts with Placebo in Reducing Menopausal Symptoms: A Randomized Double-Blind Study