Endocrinol Metab.
2019 Dec;34(4):390-397. 10.3803/EnM.2019.34.4.390.
Association between Serum Gamma-Glutamyltransferase and Prevalence of Metabolic Syndrome Using Data from the Korean Genome and Epidemiology Study
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Department of Preventive Medicine and Institute of Occupational Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. kohhj@yonsei.ac.kr
- 3Institute of Genomic Cohort, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Center for Global Health and Social Medicine, Institute of Poverty Alleviation and International Development, Yonsei University, Seoul, Korea.
- KMID: 2466258
- DOI: http://doi.org/10.3803/EnM.2019.34.4.390
Abstract
- BACKGROUND
The aim of this study was to determine whether there is a positive correlation between gamma-glutamyltransferase (GGT) levels and the prevalence of metabolic syndrome and whether GGT can be used as an easily checkable metabolic index using data from the large-scale Korean Genome and Epidemiology Study (KoGES).
METHODS
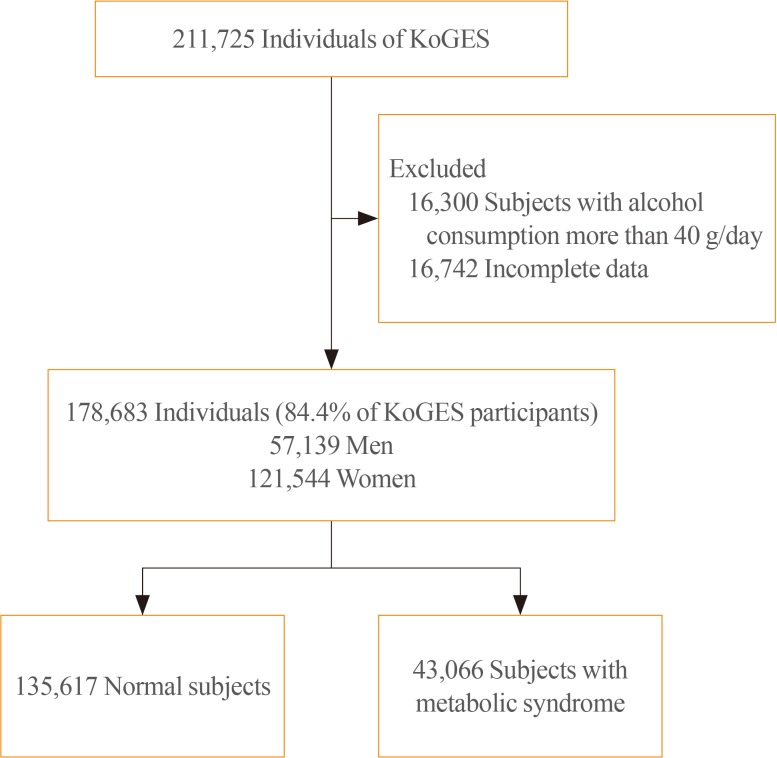
We obtained data of 211,725 participants of the KoGES. The collected data included age, sex, height, weight, waist circumference, and various biochemical characteristics, including serum GGT levels. The data of study participants who ingested more than 40 g/day of alcohol and who were diagnosed with metabolic syndrome at baseline was excluded. We analyzed the prevalence of metabolic syndrome according to GGT quartiles in both genders.
RESULTS
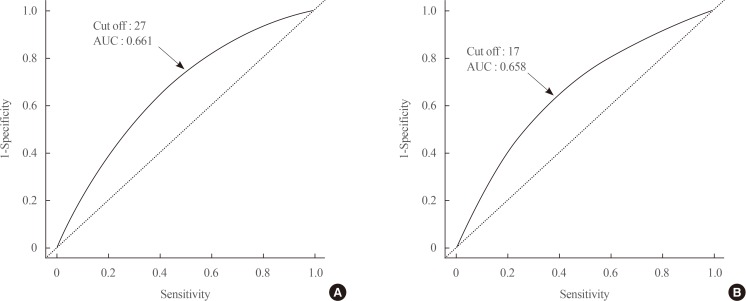
The GGT level was significantly higher in subjects with metabolic syndrome compared to normal subjects (37.92±48.20 mg/dL vs. 25.62±33.56 mg/dL). The prevalence of metabolic syndrome showed a stepwise increase with GGT quartiles in both male and female subjects. Compared to the lowest GGT quartile, the odds ratio was 1.534 (95% confidence interval [CI], 1.432 to 1.643), 1.939 (95% CI, 1.811 to 2.076), and 2.754 (95% CI, 2.572 to 2.948) in men and 1.155 (95% CI, 1.094 to 1.218), 1.528 (95% CI, 1.451 to 1.609), and 2.022 (95% CI, 1.921 to 2.218) in women with increasing GGT quartile. The cutoff value of GGT predicting risk of metabolic syndrome was 27 IU/L in men and 17 IU/L in women.
CONCLUSION
We suggested that GGT could be an easily checkable marker for the prediction of metabolic syndrome.
MeSH Terms
Figure
Reference
-
1. Teschke R, Brand A, Strohmeyer G. Induction of hepatic microsomal gamma-glutamyltransferase activity following chronic alcohol consumption. Biochem Biophys Res Commun. 1977; 75:718–724. PMID: 16594.
Article2. Sharpe PC, McBride R, Archbold GP. Biochemical markers of alcohol abuse. QJM. 1996; 89:137–144. PMID: 8729555.
Article3. Lee DH, Silventoinen K, Jacobs DR Jr, Jousilahti P, Tuomileto J. Gamma-glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab. 2004; 89:5410–5414. PMID: 15531490.4. Kim DJ, Noh JH, Cho NH, Lee BW, Choi YH, Jung JH, et al. Serum gamma-glutamyltransferase within its normal concentration range is related to the presence of diabetes and cardiovascular risk factors. Diabet Med. 2005; 22:1134–1140. PMID: 16108838.
Article5. Ndrepepa G, Braun S, Cassese S, Fusaro M, Laugwitz KL, Schunkert H, et al. Relation of gamma-glutamyl transferase to cardiovascular events in patients with acute coronary syndromes. Am J Cardiol. 2016; 117:1427–1432. PMID: 26956636.
Article6. Williams KH, Sullivan DR, Nicholson GC, George J, Jenkins AJ, Januszewski AS, et al. Opposite associations between alanine aminotransferase and γ-glutamyl transferase levels and all-cause mortality in type 2 diabetes: analysis of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Metabolism. 2016; 65:783–793. PMID: 27085785.
Article7. Lee MY, Koh SB, Koh JH, Nam SM, Shin JY, Shin YG, et al. Relationship between gamma-glutamyltransferase and metabolic syndrome in a Korean population. Diabet Med. 2008; 25:469–475. PMID: 18346161.8. Andre P, Balkau B, Vol S, Charles MA, Eschwege E. DESIR Study Group. Gamma-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged men and women: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care. 2007; 30:2355–2361. PMID: 17586745.9. Yadav D, Lee MY, Kim JY, Ryu H, Huh JH, Bae KS, et al. Combined effect of initial and longitudinal increases in γ-glutamyltransferase on incident metabolic syndrome: ARIRANG Study. Yonsei Med J. 2017; 58:763–769. PMID: 28540989.
Article10. Bo S, Gambino R, Durazzo M, Guidi S, Tiozzo E, Ghione F, et al. Associations between gamma-glutamyl transferase, metabolic abnormalities and inflammation in healthy subjects from a population-based cohort: a possible implication for oxidative stress. World J Gastroenterol. 2005; 11:7109–7117. PMID: 16437656.11. Lafontan M, Viguerie N. Role of adipokines in the control of energy metabolism: focus on adiponectin. Curr Opin Pharmacol. 2006; 6:580–585. PMID: 16973420.
Article12. Lee MY, Weon CS, Ko CH, Lee BJ, Lee Y, Kim MJ, et al. Relations between serum gamma-glutamyltransferase and prevalence of diabetes mellitus. Korean J Med. 2004; 67:498–505.13. Kim Y, Han BG. KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol. 2017; 46:e20. PMID: 27085081.
Article14. Kim J, Kim Y, Ahn YO, Paik HY, Ahn Y, Tokudome Y, et al. Development of a food frequency questionnaire in Koreans. Asia Pac J Clin Nutr. 2003; 12:243–250. PMID: 14505984.15. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007; 61:1435–1441. PMID: 17299477.
Article16. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006; 23:469–480. PMID: 16681555.17. American Heart Association. National Heart, Lung, and Blood Institue. Grundy SM, Cleeman JI, Daniels SR, Donato KA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005; 13:322–327. PMID: 16708441.18. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. PMID: 11368702.19. Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in Korean adults. Endocrinol Metab (Seoul). 2014; 29:418–426. PMID: 25559570.
Article20. Breitling LP, Raum E, Muller H, Rothenbacher D, Brenner H. Synergism between smoking and alcohol consumption with respect to serum gamma-glutamyltransferase. Hepatology. 2009; 49:802–808. PMID: 19152425.
Article21. Ferrannini E, Balkau B, Coppack SW, Dekker JM, Mari A, Nolan J, et al. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab. 2007; 92:2885–2892. PMID: 17504904.
Article22. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999; 257:79–83. PMID: 10092513.
Article23. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000; 102:1296–1301. PMID: 10982546.24. Nakanishi N, Suzuki K, Tatara K. Serum gamma-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004; 27:1427–1432. PMID: 15161799.25. Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, et al. Liver enzymes, the metabolic syndrome, and incident diabetes: the Mexico City diabetes study. Diabetes Care. 2005; 28:1757–1762. PMID: 15983331.26. Lee JH, Um MH, Park YK. The association of metabolic syndrome and serum γ-glutamyl transpeptidase: a 4-year cohort study of 3,698 Korean male workers. Clin Nutr Res. 2013; 2:67–75. PMID: 23429457.
Article27. Ndrepepa G, Kastrati A. Gamma-glutamyl transferase and cardiovascular disease. Ann Transl Med. 2016; 4:481. PMID: 28149843.
Article28. Kunutsor SK, Apekey TA, Seddoh D. Gamma glutamyltransferase and metabolic syndrome risk: a systematic review and dose-response meta-analysis. Int J Clin Pract. 2015; 69:136–144. PMID: 25363194.
Article29. Liu CF, Zhou WN, Fang NY. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract. 2012; 66:692–698. PMID: 22698421.
Article30. Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR Jr, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med. 2004; 37:1018–1023. PMID: 15336318.31. Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006; 55:928–934. PMID: 16784966.
Article32. Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004; 84:1381–1478. PMID: 15383655.
Article33. Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011; 15:1911–1926. PMID: 21126197.
Article34. Cannizzo B, Lujan A, Estrella N, Lembo C, Cruzado M, Castro C. Insulin resistance promotes early atherosclerosis via increased proinflammatory proteins and oxidative stress in fructose-fed ApoE-KO mice. Exp Diabetes Res. 2012; 2012:941304. PMID: 22474431.
Article35. Venturini D, Simao AN, Scripes NA, Bahls LD, Melo PA, Belinetti FM, et al. Evaluation of oxidative stress in overweight subjects with or without metabolic syndrome. Obesity (Silver Spring). 2012; 20:2361–2366. PMID: 22592332.
Article36. Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015; 6:109–120. PMID: 25821639.
Article37. Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers. 2015; 2015:818570. PMID: 26543300.
Article38. Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016; 31:936–944. PMID: 26667191.
Article39. Sookoian S, Pirola CJ. NAFLD. Metabolic make-up of NASH: from fat and sugar to amino acids. Nat Rev Gastroenterol Hepatol. 2014; 11:205–207. PMID: 24566880.40. Banderas DZ, Escobedo J, Gonzalez E, Liceaga MG, Ramirez JC, Castro MG. γ-Glutamyl transferase: a marker of nonalcoholic fatty liver disease in patients with the metabolic syndrome. Eur J Gastroenterol Hepatol. 2012; 24:805–810. PMID: 22546752.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between serum gamma-glutamyltransferase and metabolic syndrome among Korean non-diabetic adults
- The Association between Serum Gamma-Glutamyltransferase within Normal Levels and Metabolic Syndrome in Office Workers: A 4-Year Follow-up Study
- The Association between Gamma-glutamyltransferase and Metabolic Syndrome in Korean Male Workers
- Relationship between Serum Gamma-glutamyltransferase Levels within Reference Intervals and the Prevalence of Metabolic Syndrome and Diabetes Mellitus in Adults
- Relations of Serum gamma-glutamyltransferase Levels to Incidence of the Metabolic Syndrome