J Gastric Cancer.
2019 Dec;19(4):408-416. 10.5230/jgc.2019.19.e40.
Choice of Capecitabine or S1 in Combination with Oxaliplatin based on Thymidine Phosphorylase and Dihydropyrimidine Dehydrogenase Expression Status in Patients with Advanced Gastric Cancer
- Affiliations
-
- 1Department of Oncology, People's Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China. lv38cy@163.com
- 2Department of Hepatopathy, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China.
- KMID: 2466029
- DOI: http://doi.org/10.5230/jgc.2019.19.e40
Abstract
- PURPOSE
To study the efficacy of capecitabine or S-1 plus oxaliplatin (CAPOX or SOX) for treating thymidine phosphorylase (TP)- or dihydropyrimidine dehydrogenase (DPD)-positive advanced gastric cancer.
MATERIALS AND METHODS
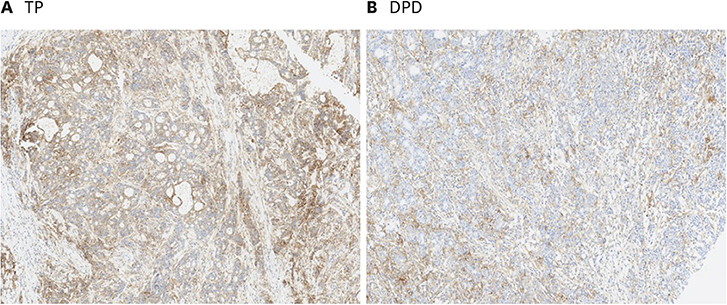
Eighty-six patients with stage IIIC to IV gastric cancer were assessed for TP and DPD expression by immunohistochemistry. The association between CAPOX or SOX efficacy and TP/DPD expression was retrospectively analyzed.
RESULTS
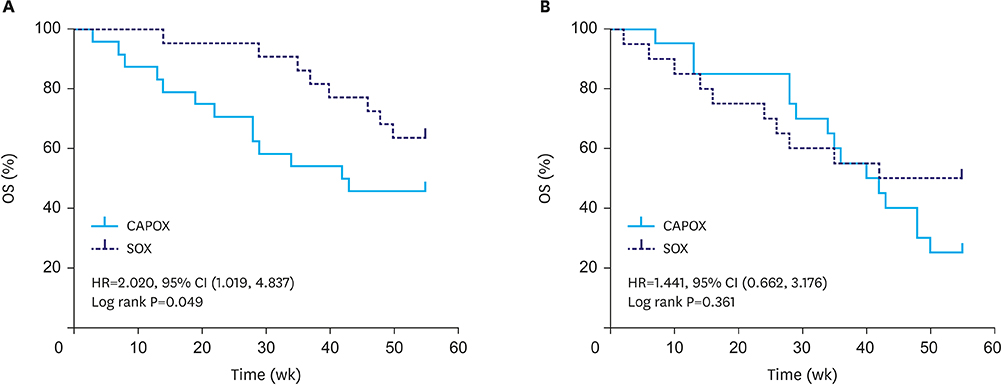
There were no significant differences in the objective remission rate (ORR, 52.27% vs. 47.62%; P>0.05), disease control rate (72.73% vs. 73.81%, P>0.05), progression-free survival (hazard ratio [HR], 1.119; 95% confidence interval [CI], 0.739-1.741; P=0.586), and overall survival (OS; HR, 0.855; 95% CI, 0.481-1.511; P=0.588) between CAPOX and SOX. A higher number of stage IV patients showed TP positivity, while DPD-positive patients predominantly showed intestinal type of gastric cancer. In TP-positive patients, the ORRs associated with CAPOX and SOX treatments were 57.14% and 38.10%, respectively; OS was better with CAPOX than with SOX (HR, 0.447; 95% CI, 0.179-0.978; P=0.046). Among DPD-positive patients, the SOX treatment-associated ORR (60.87%) was significantly higher than the CAPOX treatment-associated ORR (43.48%). Furthermore, SOX treatment resulted in better OS than did CAPOX treatment (HR, 2.020; 95% CI, 1.019-4.837; P=0.049).
CONCLUSIONS
No significant difference in clinical efficacy was found between CAPOX and SOX. TP-positive patients might respond better to CAPOX while DPD-positive patients may respond better to SOX. Our findings might serve as a guide for personalized chemotherapy for gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996; 7:548–557.2. Zhao HY, Huang H, Hu ZH, Huang Y, Lin SX, Tian Y, et al. Evaluations of biomarkers associated with sensitivity to 5-fluorouracil and taxanes for recurrent/advanced breast cancer patients treated with capecitabine-based first-line chemotherapy. Anticancer Drugs. 2012; 23:534–542.3. Ishikawa Y, Kubota T, Otani Y, Watanabe M, Teramoto T, Kumai K, et al. Dihydropyrimidine dehydrogenase activity and messenger RNA level may be related to the antitumor effect of 5-fluorouracil on human tumor xenografts in nude mice. Clin Cancer Res. 1999; 5:883–889.4. Fujiwara H, Terashima M, Irinoda T, Takagane A, Abe K, Kashiwaba M, et al. Quantitative measurement of thymidylate synthase and dihydropyrimidine dehydrogenase mRNA level in gastric cancer by real-time RT-PCR. Jpn J Cancer Res. 2002; 93:1342–1350.5. Fukuda H, Takiguchi N, Koda K, Oda K, Seike K, Miyazaki M. Thymidylate synthase and dihydropyrimidine dehydrogenase are related to histological effects of 5-fluorouracil and cisplatin neoadjuvant chemotherapy for primary gastric cancer patients. Cancer Invest. 2006; 24:235–241.6. Takabayashi A, Iwata S, Kawai Y, Kanai M, Taki Y, Takechi T, et al. Dihydropyrimidine dehydrogenase activity and mRNA expression in advanced gastric cancer analyzed in relation to effectiveness of preoperative 5-fluorouracil-based chemotherapy. Int J Oncol. 2000; 17:889–895.7. Ishikawa Y, Kubota T, Otani Y, Watanabe M, Teramoto T, Kumai K, et al. Dihydropyrimidine dehydrogenase and messenger RNA levels in gastric cancer: possible predictor for sensitivity to 5-fluorouracil. Jpn J Cancer Res. 2000; 91:105–112.8. Terashima M, Fujiwara H, Takagane A, Abe K, Irinoda T, Nakaya T, et al. Prediction of sensitivity to fluoropyrimidines by metabolic and target enzyme activities in gastric cancer. Gastric Cancer. 2003; 6:Suppl 1. 71–81.9. Oeda M, Yoshida K, Sanada Y, Wada Y, Suzuki T, Mizuiri H, et al. The expression profiles of orotate phosphoribosyltransferase and dihydropyrimidine dehydrogenase in gastric cancer and their clinical significance. Oncol Rep. 2006; 16:1165–1172.10. Koizumi W, Okayasu I, Hyodo I, Sakamoto J, Kojima H. Clinical Study Group of Capecitabine. Prediction of the effect of capecitabine in gastric cancer by immunohistochemical staining of thymidine phosphorylase and dihydropyrimidine dehydrogenase. Anticancer Drugs. 2008; 19:819–824.11. Maehara Y. S-1 in gastric cancer: a comprehensive review. Gastric Cancer. 2003; 6:Suppl 1. 2–8.12. Yin J, Song JN, Bai ZG, Cai J, Zhang J, Zheng Z, et al. Gastric cancer mortality trends in China (2006-2013) reveal increasing mortality in young subjects. Anticancer Res. 2017; 37:4671–4679.13. Li M, Wan X, Wang Y, Sun Y, Yang G, Wang L. Time trends of esophageal and gastric cancer mortality in China, 1991-2009: an age-period-cohort analysis. Sci Rep. 2017; 7:6797.14. Aprile G, Mazzer M, Moroso S, Puglisi F. Pharmacology and therapeutic efficacy of capecitabine: focus on breast and colorectal cancer. Anticancer Drugs. 2009; 20:217–229.15. Toi M, Atiqur Rahman M, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005; 6:158–166.16. Wang D, Yu X, Wang X. High/positive expression of 5-fluorouracil metabolic enzymes predicts better response to S-1 in patients with gastric cancer: a meta-analysis. Int J Biol Markers. 2016; 31:e101–e109.17. Iwahashi S, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, et al. Role of thymidylate synthase and dihydropyrimidine dehydrogenase mRNA expressions in gallbladder carcinoma. Surg Today. 2012; 42:565–569.18. Jeung HC, Rha SY, Shin SJ, Lim SJ, Roh JK, Noh SH, et al. Predictive values of 5-fluorouracil pathway genes for S-1 treatment in patients with advanced gastric cancer. Anticancer Drugs. 2011; 22:801–810.19. Yang Q, Barbareschi M, Mori I, Mauri F, Muscarà M, Nakamura M, et al. Prognostic value of thymidine phosphorylase expression in breast carcinoma. Int J Cancer. 2002; 97:512–517.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Schedule Dependency for Induction of Thymidine Phosphorylase Activity and the Enhancement of Capecitabine Efficacy by Docetaxel on the SNU-484 Gastric Cancer Cell Line That was Injected into Xenografted Nude Mice
- Capecitabine Plus Oxaliplatin Combination Therapy for Basal Cell Carcinoma
- Development of inhibitors of pyrimidine metabolism
- Capecitabine and Oxaliplatin (XELOX) for the Treatment of Patients with Metastatic Gastric Cancer and Severe Liver Dysfunction
- Expression of thymidine phosphorylase in cervical neoplasia: correlation with clinicopathological prognostic factors