Ann Lab Med.
2020 May;40(3):253-258. 10.3343/alm.2020.40.3.253.
Isolation of Small Extracellular Vesicles From Human Serum Using a Combination of Ultracentrifugation With Polymer-Based Precipitation
- Affiliations
-
- 1Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology, Sungkyunkwan University, Seoul, Korea. kstwoh@skku.edu
- 2Samsung Biomedical Research Institute, Samsung Medical Center, Seoul, Korea.
- 3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2466019
- DOI: http://doi.org/10.3343/alm.2020.40.3.253
Abstract
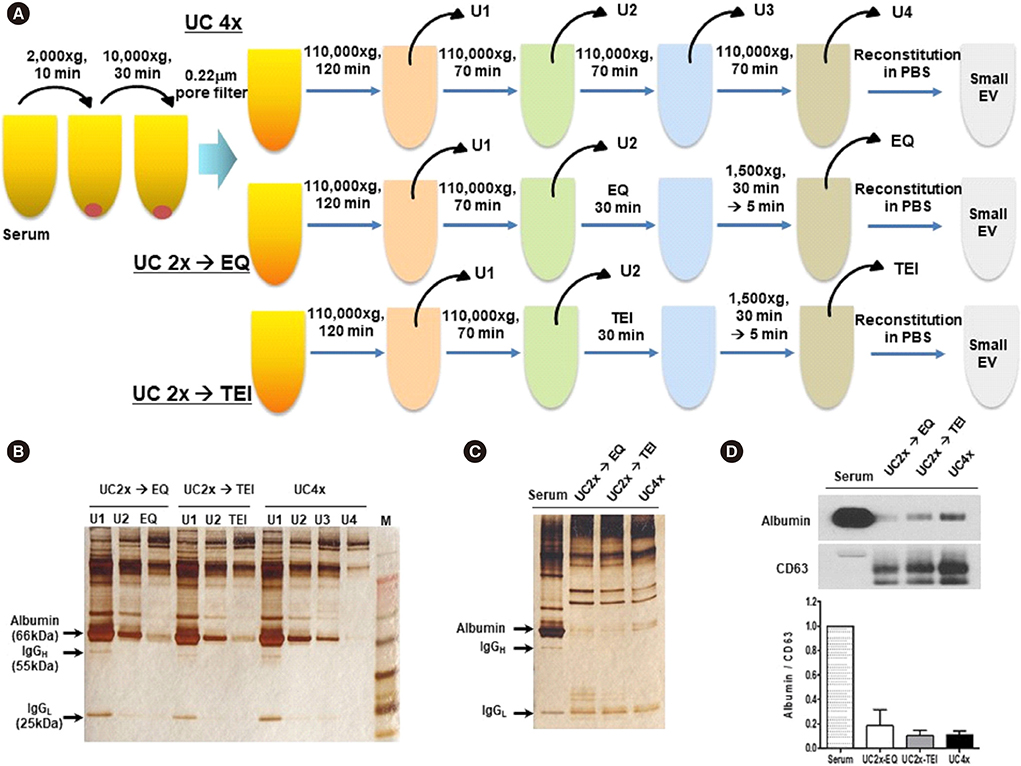
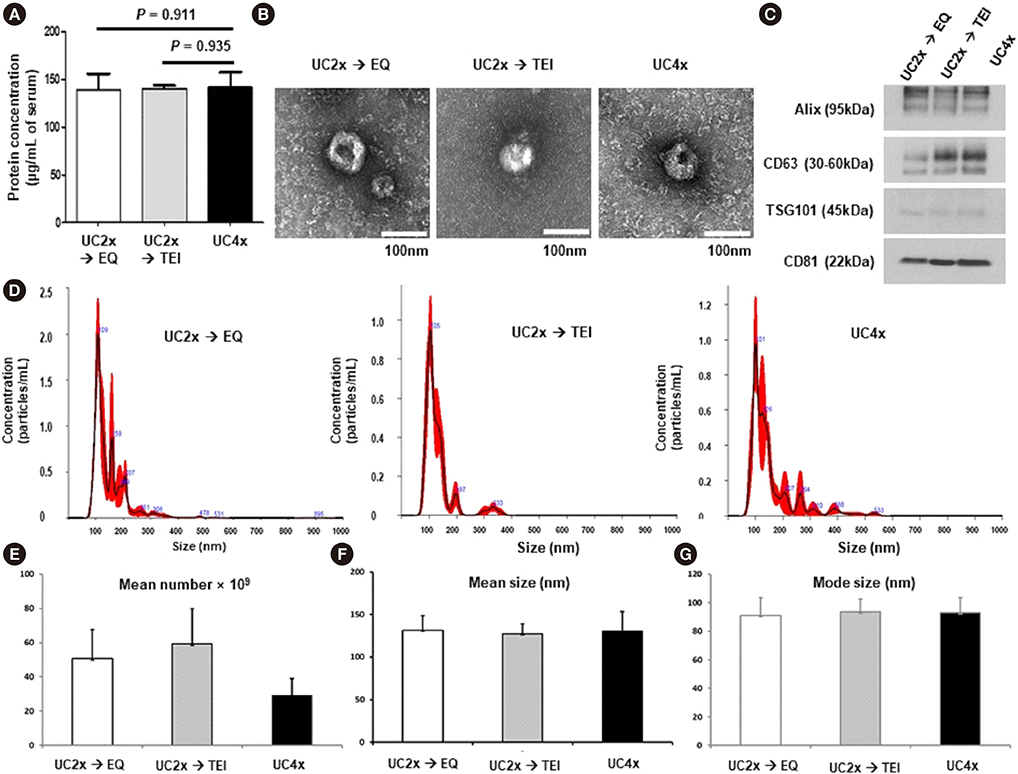
- Methods for reproducibly isolating and enriching small extracellular vesicles (EVs) from blood are essential for clinical utilization of small EVs in cancer patients. We combined ultracentrifugation (UC) with polymer-based precipitation (ExoQuick [EQ] or Total Exosome Isolation [TEI] kit) to isolate small EVs (diameter, 30-150 nm) from the serum of breast cancer patients. We compared the performance of four cycles of UC (UC4x) with that of two cycles of UC followed by enrichment using the EQ (UC2x→EQ) or TEI (UC2x→TEI) kits. The mean concentration of small EVs isolated from 1 mL of serum using UC2x→EQ (139.0±29.1 µg) and UC2x→TEI (140.4±5.0 µg) did not differ from that obtained using UC4x (141.8±26.9 µg). The mean number of EV particles obtained using UC4x was 29.2±9.9×109 per mL of serum, whereas UC2x→EQ and UC2x→TEI yielded higher numbers of EVs (50.7±17.0×10â¹ and 59.3±20.6×10â¹, respectively). Concentrations of EV microRNAs, including miR-21 and miR-155, did not differ between the three methods. In conclusion, performing UC prior to the use of polymer-based precipitation kits could be feasible for isolating small EVs from human serum in large sample-based translational researches.
MeSH Terms
Figure
Reference
-
1. Sharma A, Khatun Z, Shiras A. Tumor exosomes: cellular postmen of cancer diagnosis and personalized therapy. Nanomedicine (Lond). 2016; 11:421–437.
Article2. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013; 12:347–357.
Article3. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016; 164:1226–1232.
Article4. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015; 25:364–372.
Article5. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200:373–383.
Article6. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018; 7:1535750.7. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008; 10:1470–1476.
Article8. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015; 523:177–182.
Article9. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017; 7:789–804.
Article10. Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015; 4:27031.
Article11. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014; 3:26913.
Article12. Koh YQ, Almughlliq FB, Vaswani K, Peiris HN, Mitchell MD. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (Landmark Ed). 2018; 23:865–874.13. An M, Wu J, Zhu J, Lubman DM. Comparison of an optimized ultracentrifugation method versus size-exclusion chromatography for isolation of exosomes from human serum. J Proteome Res. 2018; 17:3599–3605.
Article14. Yamada T, Inoshima Y, Matsuda T, Ishiguro N. Comparison of methods for isolating exosomes from bovine milk. J Vet Med Sci. 2012; 74:1523–1525.
Article15. Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014; 26:707–721.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular Vesicles in Psychiatry Research in the Context of RDoC Criteria
- Urinary Extracellular Vesicles as Biomarkers of Kidney Disease
- Extracellular Vesicles, a Key Mediator to Link Environmental Microbiota to Airway Immunity
- Extracellular Vesicles and Immune System in Ageing and Immune Diseases
- Microbe-derived extracellular vesicles as a smart drug delivery system