Ann Lab Med.
2020 May;40(3):245-252. 10.3343/alm.2020.40.3.245.
Reporting Quality of Diagnostic Accuracy Studies in Laboratory Medicine: Adherence to Standards for Reporting of Diagnostic Accuracy Studies (STARD) 2015
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. cecilia@schmc.ac.kr
- 2Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2466018
- DOI: http://doi.org/10.3343/alm.2020.40.3.245
Abstract
- BACKGROUND
Poor reporting quality in diagnostic accuracy studies hampers an adequate judgment of the validity of the study. The Standards for Reporting of Diagnostic Accuracy Studies (STARD) statement was published to improve the reporting quality of diagnostic accuracy studies. This study aimed to evaluate the adherence of diagnostic accuracy studies published in Annals of Laboratory Medicine (ALM) to STARD 2015 and to identify directions for improvement in the reporting quality of these studies.
METHODS
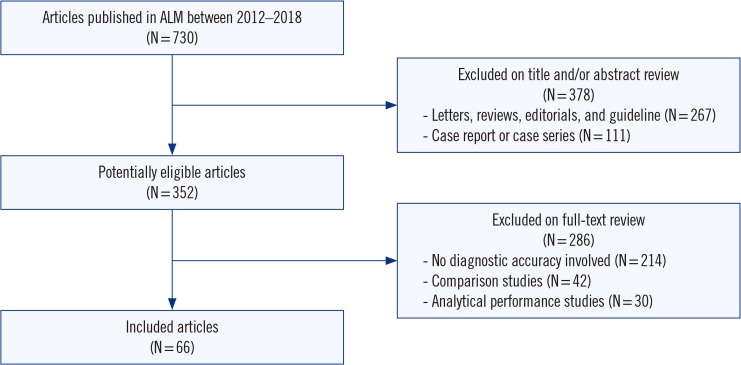
Two independent authors assessed articles published in ALM between 2012-2018 for compliance with 30 STARD 2015 checklist items to identify all eligible diagnostic accuracy studies published during this period. We included 66 diagnostic accuracy studies. A total of the fulfilled STARD items were calculated, and adherence was analyzed on an individual-item basis.
RESULTS
The overall mean±SD number of STARD items reported for the included studies was 11.2±2.7. Only five (7.6%) studies adhered to more than 50% of the 30 items. No study satisfied more than 80% of the items. Large variability in adherence to reporting standards was detected across items, ranging from 0% to 100%.
CONCLUSIONS
Adherence to STARD 2015 is suboptimal among diagnostic accuracy studies published in ALM. Our study emphasizes the necessity of adherence to STARD to improve the reporting quality of future diagnostic accuracy studies to be published in ALM.
Keyword
Figure
Reference
-
1. Kosack CS, Page AL, Klatser PR. A guide to aid the selection of diagnostic tests. Bull World Health Organ. 2017; 95:639–645. PMID: 28867844.2. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015; 351:h5527. PMID: 26511519.3. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Clin Chem. 2015; 61:1446–1452. PMID: 26510957.4. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology. 2015; 277:826–832. PMID: 26509226.5. Schmidt RL, Factor RE. Understanding sources of bias in diagnostic accuracy studies. Arch Pathol Lab Med. 2013; 137:558–565. PMID: 23544945.6. Reid MC, Lachs MS, Feinstein AR. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995; 274:645–651. PMID: 7637146.7. Glasziou P, Altman DG, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014; 383:267–276. PMID: 24411647.8. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Clin Chem. 2003; 49:7–18. PMID: 12507954.9. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003; 44:635–638. PMID: 14515428.10. Korevaar DA, Wang J, van Enst WA, Leeflang MM, Hooft L, Smidt N, et al. Reporting diagnostic accuracy studies: some improvements after 10 years of STARD. Radiology. 2015; 274:781–789. PMID: 25350641.11. Korevaar DA, van Enst WA, Spijker R, Bossuyt PM, Hooft L. Reporting quality of diagnostic accuracy studies: a systematic review and meta-analysis of investigations on adherence to STARD. Evid Based Med. 2014; 19:47–54. PMID: 24368333.12. Smidt N, Rutjes AW, van der Windt DA, Ostelo RW, Bossuyt PM, Reitsma JB, et al. The quality of diagnostic accuracy studies since the STARD statement: has it improved? Neurology. 2006; 67:792–797. PMID: 16966539.13. Kim JH. Editorial announcement regarding title change of the Korean Journal of Laboratory Medicine to Annals of Laboratory Medicine. Ann Lab Med. 2012; 32:1–2.14. Cohen JF, Korevaar DA, Gatsonis CA, Glasziou PP, Hooft L, Moher D, et al. STARD for Abstracts: essential items for reporting diagnostic accuracy studies in journal or conference abstracts. BMJ. 2017; 358:j3751. PMID: 28819063.15. Walther S, Schueler S, Tackmann R, Schuetz GM, Schlattmann P, Dewey M. Compliance with STARD checklist among studies of coronary CT angiography: systematic review. Radiology. 2014; 271:74–86. PMID: 24475846.16. Choi YJ, Chung MS, Koo HJ, Park JE, Yoon HM, Park SH. Does the reporting quality of diagnostic test accuracy studies, as defined by STARD 2015, affect citation? Korean J Radiol. 2016; 17:706–714. PMID: 27587959.17. Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016; 6:e012799.18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174. PMID: 843571.19. Michelessi M, Lucenteforte E, Miele A, Oddone F, Crescioli G, Fameli V, et al. Diagnostic accuracy research in glaucoma is still incompletely reported: an application of Standards for Reporting of Diagnostic Accuracy Studies (STARD) 2015. PLoS One. 2017; 12:e0189716. PMID: 29240827.20. Hong PJ, Korevaar DA, McGrath TA, Ziai H, Frank R, Alabousi M, et al. Reporting of imaging diagnostic accuracy studies with focus on MRI subgroup: adherence to STARD 2015. J Magn Reson Imaging. 2018; 47:523–544. PMID: 28640484.21. Fontela PS, Pant Pai N, Schiller I, Dendukuri N, Ramsay A, Pai M. Quality and reporting of diagnostic accuracy studies in TB, HIV and malaria: evaluation using QUADAS and STARD standards. PLoS One. 2009; 4:e7753. PMID: 19915664.22. Whiting PF, Rutjes AW, Westwood ME, Mallett S. QUADAS-2 Steering Group. A systematic review classifies sources of bias and variation in diagnostic test accuracy studies. J Clin Epidemiol. 2013; 66:1093–1104. PMID: 23958378.23. Shinkins B, Thompson M, Mallett S, Perera R. Diagnostic accuracy studies: how to report and analyse inconclusive test results. BMJ. 2013; 346:f2778. PMID: 23682043.24. Naaktgeboren CA, de Groot JA, Rutjes AW, Bossuyt PM, Reitsma JB, Moons KG. Anticipating missing reference standard data when planning diagnostic accuracy studies. BMJ. 2016; 352:i402. PMID: 26861453.25. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536. PMID: 22007046.26. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919. PMID: 27733354.27. Cha YJ, Park Q, Kang ES, Yoo BC, Park KU, Kim JW, et al. Performance evaluation of the OraQuick hepatitis C virus rapid antibody test. Ann Lab Med. 2013; 33:184–189. PMID: 23667844.28. Lee J, Lee HS, Cho YG, Choi SI, Kim DS. Evaluation of Allplex Respiratory Panel 1/2/3 multiplex real-time PCR assays for the detection of respiratory viruses with influenza A virus subtyping. Ann Lab Med. 2018; 38:46–50. PMID: 29071818.29. Hwang Y, Lee M. Comparison of the AdvanSure human papillomavirus screening real-time PCR, the Abbott RealTime high risk human papillomavirus test, and the Hybrid Capture human papillomavirus DNA test for the detection of human papillomavirus. Ann Lab Med. 2012; 32:201–205. PMID: 22563555.30. Gupta A, Roehrborn CG. Verification and incorporation biases in studies assessing screening tests: prostate-specific antigen as an example. Urology. 2004; 64:106–111. PMID: 15245945.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Does the Reporting Quality of Diagnostic Test Accuracy Studies, as Defined by STARD 2015, Affect Citation?
- Quality Evaluation of the Performance Study of Diagnostic Tests Using STARD Checklist and Meta-Analysis for the Pooled Sensitivity and Specificity of Third Generation Anti-HCV EIA Tests
- Meta-Analysis of Diagnostic Test Accuracy
- Overview of the Process of Conducting Meta-analyses of the Diagnostic Test Accuracy
- Recent Research Trends in Meta-analysis