Int J Stem Cells.
2019 Jul;12(2):367-379. 10.15283/ijsc18151.
Monitoring Glutathione Dynamics and Heterogeneity in Living Stem Cells
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul, Korea. igkim@plaza.snu.ac.kr
- 2BK21 Plus Biomedical Science Project, Seoul National University, Seoul, Korea.
- 3Cell2in, Inc., Seoul, Korea.
- 4Department of Biomedical Sciences, University of Ulsan College of Medicine, Seoul, Korea. d0shin03@amc.seoul.kr
- 5Department of Chemistry, Korea University, Seoul, Korea. kchoi@korea.ac.kr
- 6Metabolab. Inc., Seoul, Korea.
- KMID: 2465906
- DOI: http://doi.org/10.15283/ijsc18151
Abstract
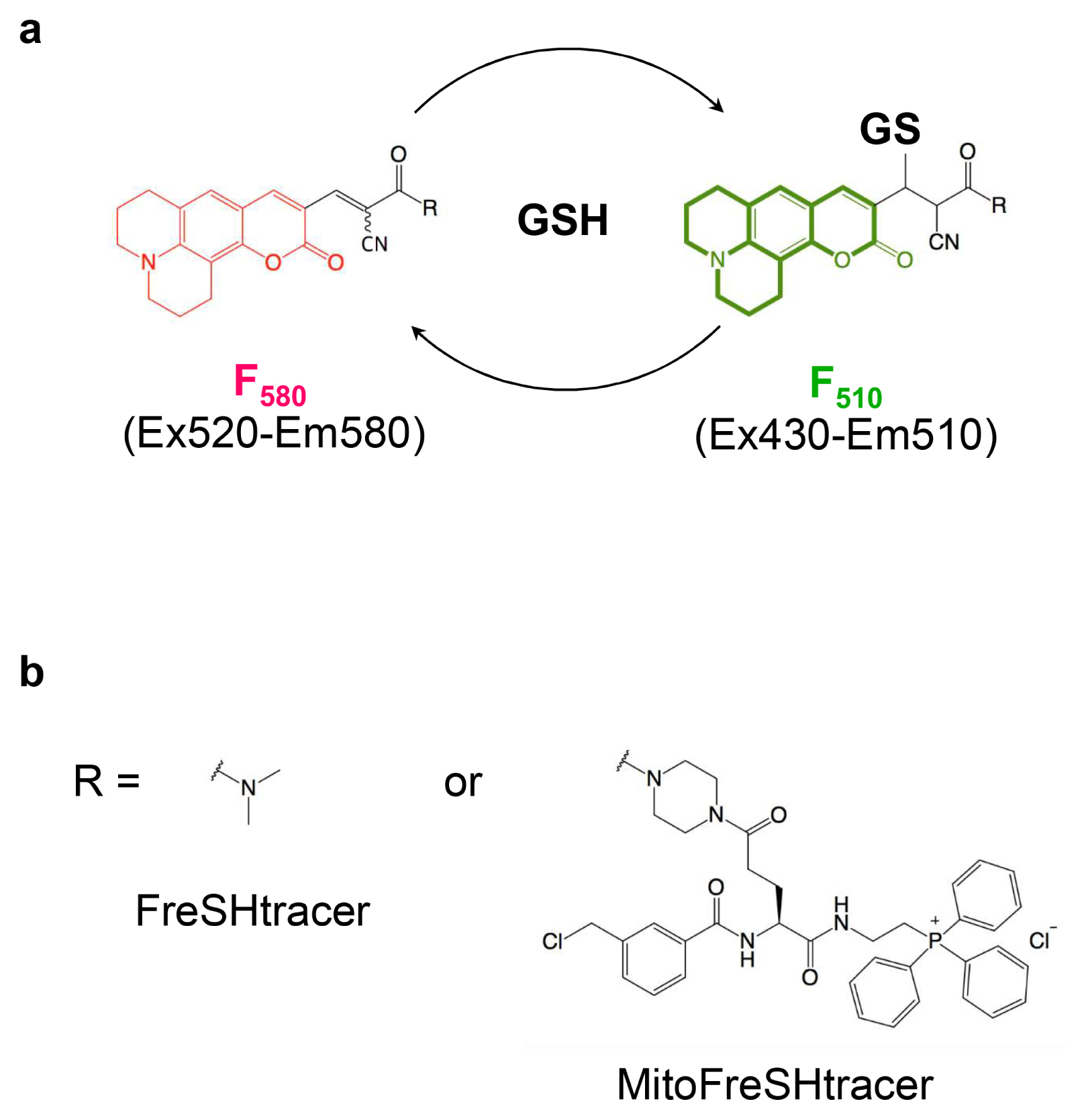
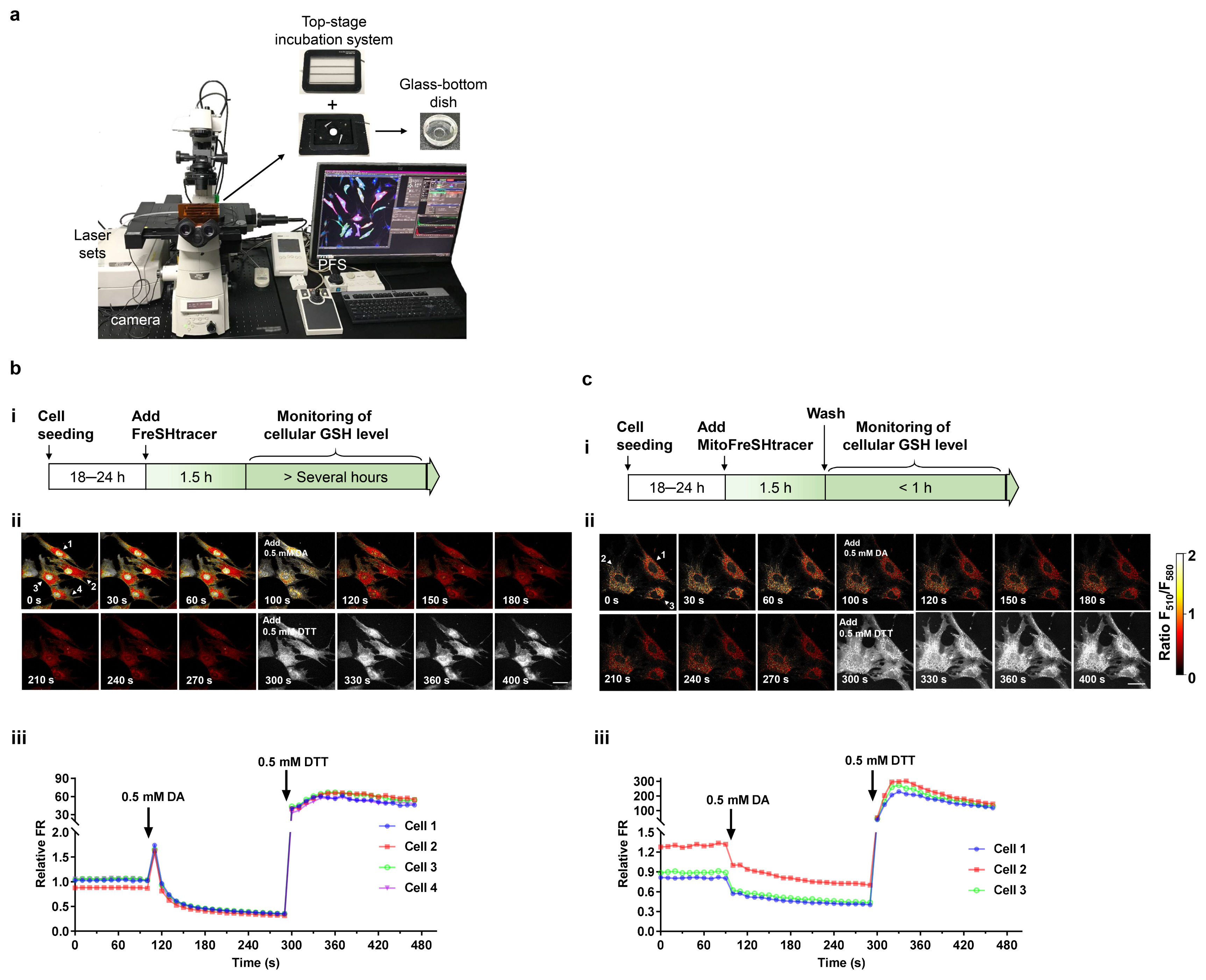
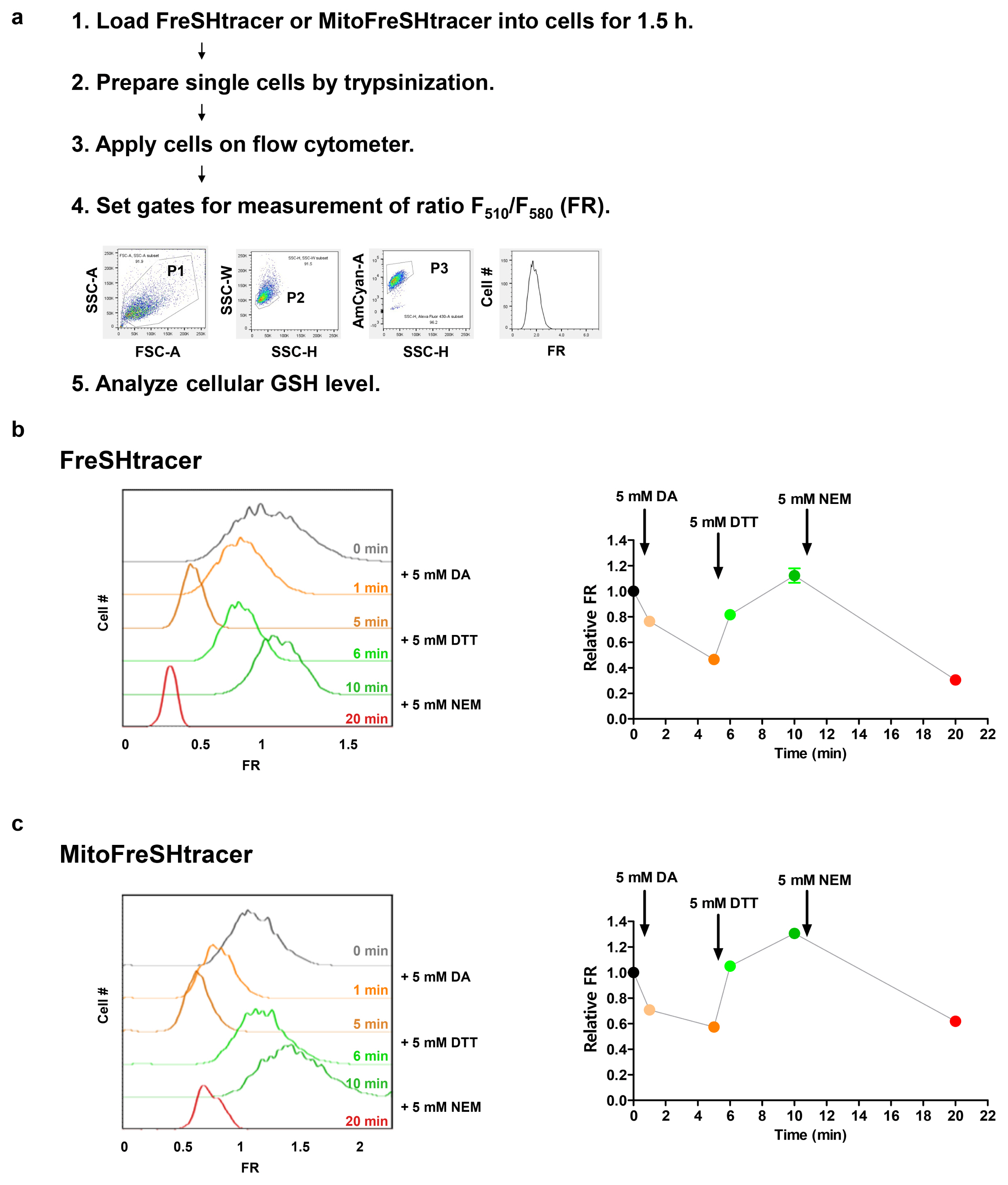
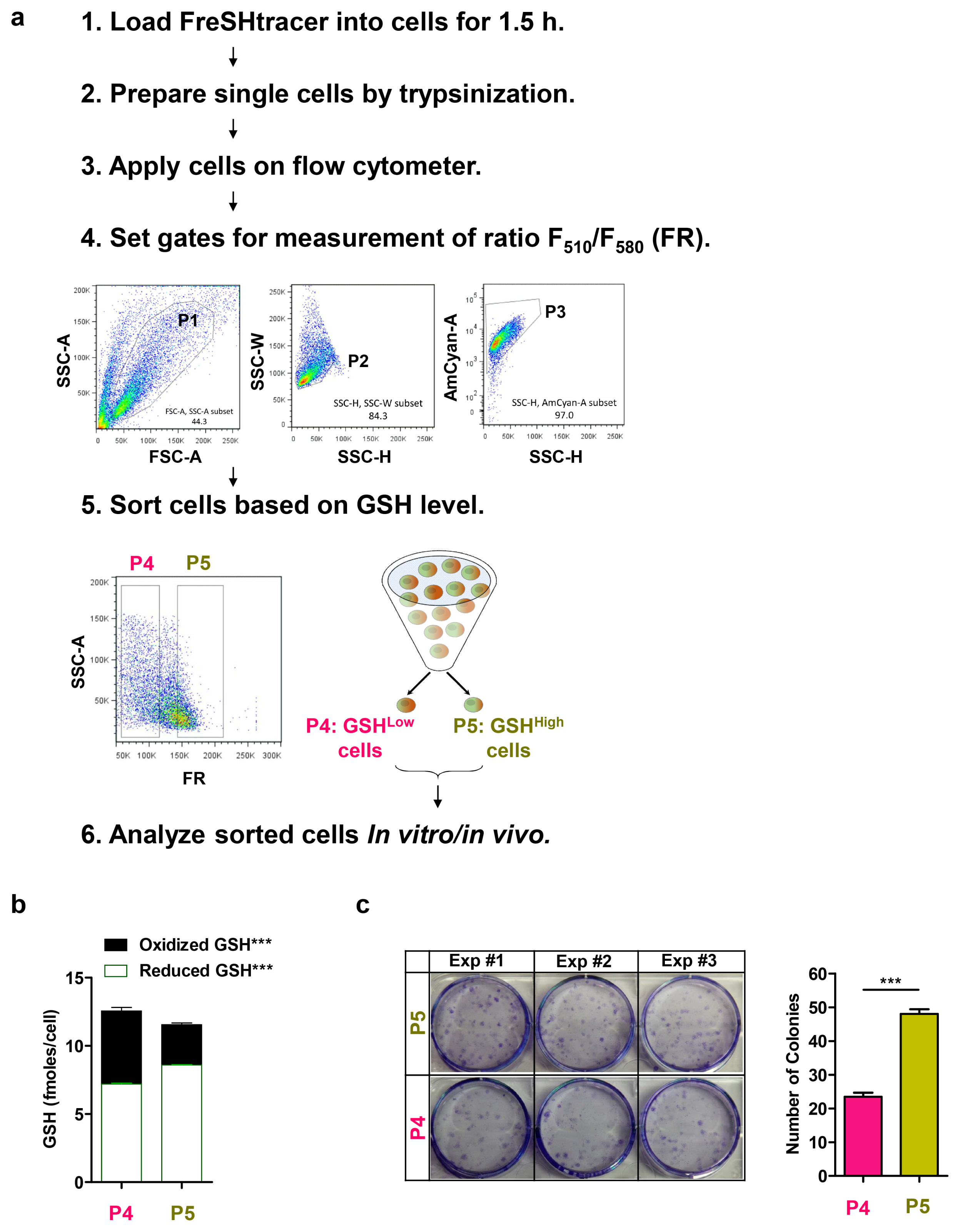
- Glutathione (GSH) is a major antioxidant in cells, and plays vital roles in the cellular defense against oxidants and in the regulation of redox signals. In a previous report, we demonstrated that stem cell function is critically affected by heterogeneity and dynamic changes in cellular GSH concentration. Here, we present a detailed protocol for the monitoring of GSH concentration in living stem cells using FreSHtracer, a real-time GSH probe. We describe the steps involved in monitoring GSH concentration in single living stem cells using confocal microscopy and flow cytometry. These methods are simple, rapid, and quantitative, and able to demonstrate intracellular GSH concentration changes in real time. We also describe the application of FreSHtracer to the sorting of stem cells according to their GSH content using flow cytometry. Typically, microscopic or flow cytometric analyses of FreSHtracer and MitoFreSHtracer signals in living stem cells take ~2~3 h, and the fractionation of stem cells into subpopulations on the basis of cellular GSH levels takes 3~4.5 h. This method could be applied to almost every kind of mammalian cell with minor modifications to the protocol described here.
MeSH Terms
Figure
Cited by 2 articles
-
Antioxidant and antiobesity activities of oral treatment with ethanol extract from sprout of evening primrose (Oenothera laciniata) in high fat diet-induced obese mice
Chung Shil Kwak, Mi-Ju Kim, Sun Gi Kim, Sunyeong Park, In Gyu Kim, Heun Soo Kang
J Nutr Health. 2019;52(6):529-539. doi: 10.4163/jnh.2019.52.6.529.Measuring Glutathione Regeneration Capacity in Stem Cells
Jihye Kim, Yi-Xi Gong, Eui Man Jeong
Int J Stem Cells. 2023;16(3):356-362. doi: 10.15283/ijsc23047.
Reference
-
References
1. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004; 134:489–492. DOI: 10.1093/jn/134.3.489. PMID: 14988435.
Article2. Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013; 1830:3143–3153. DOI: 10.1016/j.bbagen.2012.09.008. PMID: 22995213. PMCID: PMC3549305.
Article3. Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008; 45:549–561. DOI: 10.1016/j.freeradbiomed.2008.05.004. PMID: 18544350.
Article4. Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003; 57:145–155. DOI: 10.1016/S0753-3322(03)00043-X. PMID: 12818476. PMCID: PMC6522248.
Article5. Ok JS, Song SB, Hwang ES. Enhancement of replication and differentiation potential of human bone marrow stem cells by nicotinamide treatment. Int J Stem Cells. 2018; 11:13–25. DOI: 10.15283/ijsc18033. PMID: 29699388. PMCID: PMC5984055.
Article6. Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, Lee S, Lim J, Jeong SY, Kwon J, Kim M, Choi SJ, Oh W, Yang YS, Hwang HH, Yu HY, Ryu CM, Jeon HB, Shin DM. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018; 32:2672–2684. DOI: 10.1038/s41375-018-0151-8. PMID: 29789652. PMCID: PMC6286327.
Article7. Heo J, Lim J, Lee S, Jeong J, Kang H, Kim Y, Kang JW, Yu HY, Jeong EM, Kim K, Kucia M, Waigel SJ, Zacharias W, Chen Y, Kim IG, Ratajczak MZ, Shin DM. Sirt1 regulates DNA methylation and differentiation potential of embryonic stem cells by antagonizing Dnmt3l. Cell Rep. 2017; 18:1930–1945. DOI: 10.1016/j.celrep.2017.01.074. PMID: 28228259.
Article8. Lee CW, Kang D, Kim AK, Kim DY, Kim DI. Improvement of cell cycle lifespan and genetic damage susceptibility of human mesenchymal stem cells by hypoxic priming. Int J Stem Cells. 2018; 11:61–67. DOI: 10.15283/ijsc17054. PMID: 29699381. PMCID: PMC5984059.
Article9. Cho AY, Choi K. A coumarin-based fluorescence sensor for the reversible detection of thiols. Chem Lett. 2012; 41:1611–1612. DOI: 10.1246/cl.2012.1611.
Article10. Jeong EM, Yoon JH, Lim J, Shin JW, Cho AY, Heo J, Lee KB, Lee JH, Lee WJ, Kim HJ, Son YH, Lee SJ, Cho SY, Shin DM, Choi K, Kim IG. Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function. Stem Cell Reports. 2018; 10:600–614. DOI: 10.1016/j.stemcr.2017.12.007. PMID: 29307581. PMCID: PMC5830891.
Article11. Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006; 1:3159–3165. DOI: 10.1038/nprot.2006.378. PMID: 17406579.
Article12. Monostori P, Wittmann G, Karg E, Túri S. Determination of glutathione and glutathione disulfide in biological samples: an in-depth review. J Chromatogr B Analyt Technol Biomed Life Sci. 2009; 877:3331–3346. DOI: 10.1016/j.jchromb.2009.06.016. PMID: 19560987.
Article13. Kim GJ, Lee K, Kwon H, Kim HJ. Ratiometric fluorescence imaging of cellular glutathione. Org Lett. 2011; 13:2799–2801. DOI: 10.1021/ol200967w. PMID: 21548608.
Article14. Sebastià J, Cristòfol R, Martín M, Rodríguez-Farré E, Sanfeliu C. Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry A. 2003; 51:16–25. DOI: 10.1002/cyto.a.10003. PMID: 12500301.
Article15. Peng H, Chen W, Cheng Y, Hakuna L, Strongin R, Wang B. Thiol reactive probes and chemosensors. Sensors (Basel). 2012; 12:15907–15946. DOI: 10.3390/s121115907. PMID: 23202239. PMCID: PMC3522992.
Article16. Zou Y, Wang A, Shi M, Chen X, Liu R, Li T, Zhang C, Zhang Z, Zhu L, Ju Z, Loscalzo J, Yang Y, Zhao Y. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat Protoc. 2018; 13:2362–2386. DOI: 10.1038/s41596-018-0042-5. PMID: 30258175.
Article17. Chen J, Jiang X, Zhang C, MacKenzie KR, Stossi F, Palzkill T, Wang MC, Wang J. Reversible reaction-based fluorescent probe for real-time imaging of glutathione dynamics in mitochondria. ACS Sens. 2017; 2:1257–1261. DOI: 10.1021/acssensors.7b00425. PMID: 28809477. PMCID: PMC5771714.
Article18. Jiang X, Chen J, Bajić A, Zhang C, Song X, Carroll SL, Cai ZL, Tang M, Xue M, Cheng N, Schaaf CP, Li F, MacKenzie KR, Ferreon ACM, Xia F, Wang MC, Maletić-Savatić M, Wang J. Quantitative real-time imaging of glutathione. Nat Commun. 2017; 8:16087. DOI: 10.1038/ncomms16087. PMID: 28703127. PMCID: PMC5511354.
Article19. Jiang X, Yu Y, Chen J, Zhao M, Chen H, Song X, Matzuk AJ, Carroll SL, Tan X, Sizovs A, Cheng N, Wang MC, Wang J. Quantitative imaging of glutathione in live cells using a reversible reaction-based ratiometric fluorescent probe. ACS Chem Biol. 2015; 10:864–874. DOI: 10.1021/cb500986w. PMID: 25531746. PMCID: PMC4371605.
Article20. Umezawa K, Yoshida M, Kamiya M, Yamasoba T, Urano Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat Chem. 2017; 9:279–286. DOI: 10.1038/nchem.2648. PMID: 28221345.
Article21. Serafimova IM, Pufall MA, Krishnan S, Duda K, Cohen MS, Maglathlin RL, McFarland JM, Miller RM, Frödin M, Taunton J. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat Chem Biol. 2012; 8:471–476. DOI: 10.1038/nchembio.925. PMID: 22466421. PMCID: PMC3657615.
Article22. Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A. 2009; 106:422–427. DOI: 10.1073/pnas.0812149106. PMID: 19122143. PMCID: PMC2626718.
Article23. Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007; 47:629–656. DOI: 10.1146/annurev.pharmtox.47.120505.105110.
Article24. Presley AD, Fuller KM, Arriaga EA. MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 793:141–150. DOI: 10.1016/S1570-0232(03)00371-4. PMID: 12880861.
Article25. Jiang X, Zhang C, Chen J, Choi S, Zhou Y, Zhao M, Song X, Chen X, Maletic-Savatic M, Palzkill TG, Moore D, Wang MC, Wang J. Quantitative real-time imaging of glutathione with sub-cellular resolution. Antioxid Redox Signal. 2018; [Epub ahead of print]. DOI: 10.1089/ars.2018.7605. PMID: 30358421. PMCID: PMC6486671.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measuring Glutathione Regeneration Capacity in Stem Cells
- Cancer Stem Cells in Brain Tumors and Their Lineage Hierarchy
- Genetic heterogeneity of liver cancer stem cells
- Heterogeneity of Islet Cells during Embryogenesis and Differentiation
- Recent Advances for Enhancing Drug Metabolizing Functions of Hepatocyte-like Cells Derived from Human Pluripotent Stem Cells