Int J Stem Cells.
2019 Jul;12(2):315-330. 10.15283/ijsc18098.
A Novel Immunomodulatory Mechanism Dependent on Acetylcholine Secreted by Human Bone Marrow-derived Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Integrated Biomedical Sciences, Inha University School of Medicine, Incheon, Korea. sunuksong@inha.ac.kr
- 2SCM Lifescience Co., Ltd., Incheon, Korea.
- 3SunCreate Co., Ltd., Yangju, Korea.
- 4Department of Radiooncology, Inha University School of Medicine, Incheon, Korea.
- KMID: 2465901
- DOI: http://doi.org/10.15283/ijsc18098
Abstract
- BACKGROUND AND OBJECTIVES
Mesenchymal stem cells (MSCs) are used to treat autoimmune or inflammatory diseases. Our aim was to determine the immunomodulatory mechanisms elicited by MSCs during inflammation.
METHODS AND RESULTS
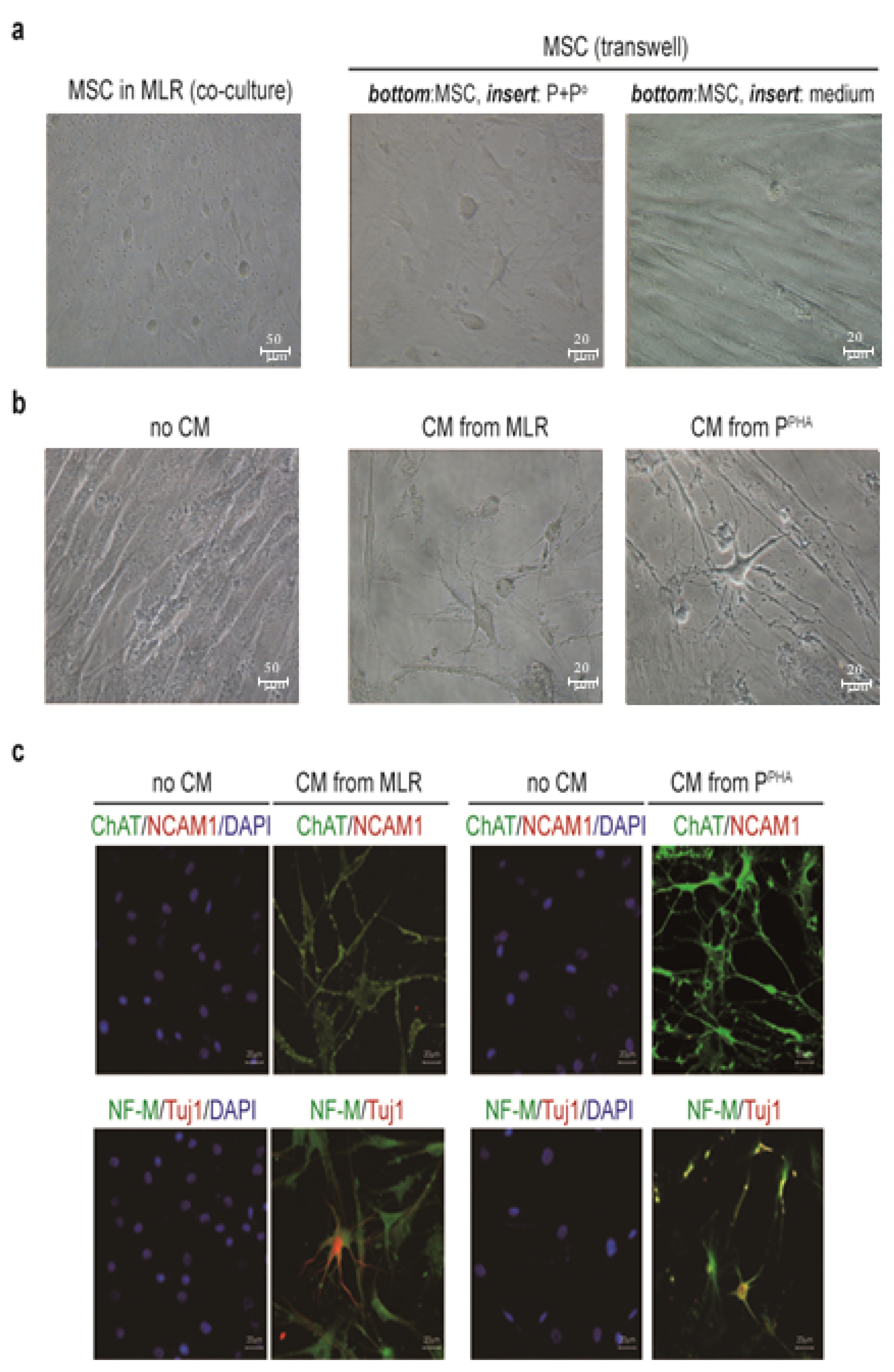
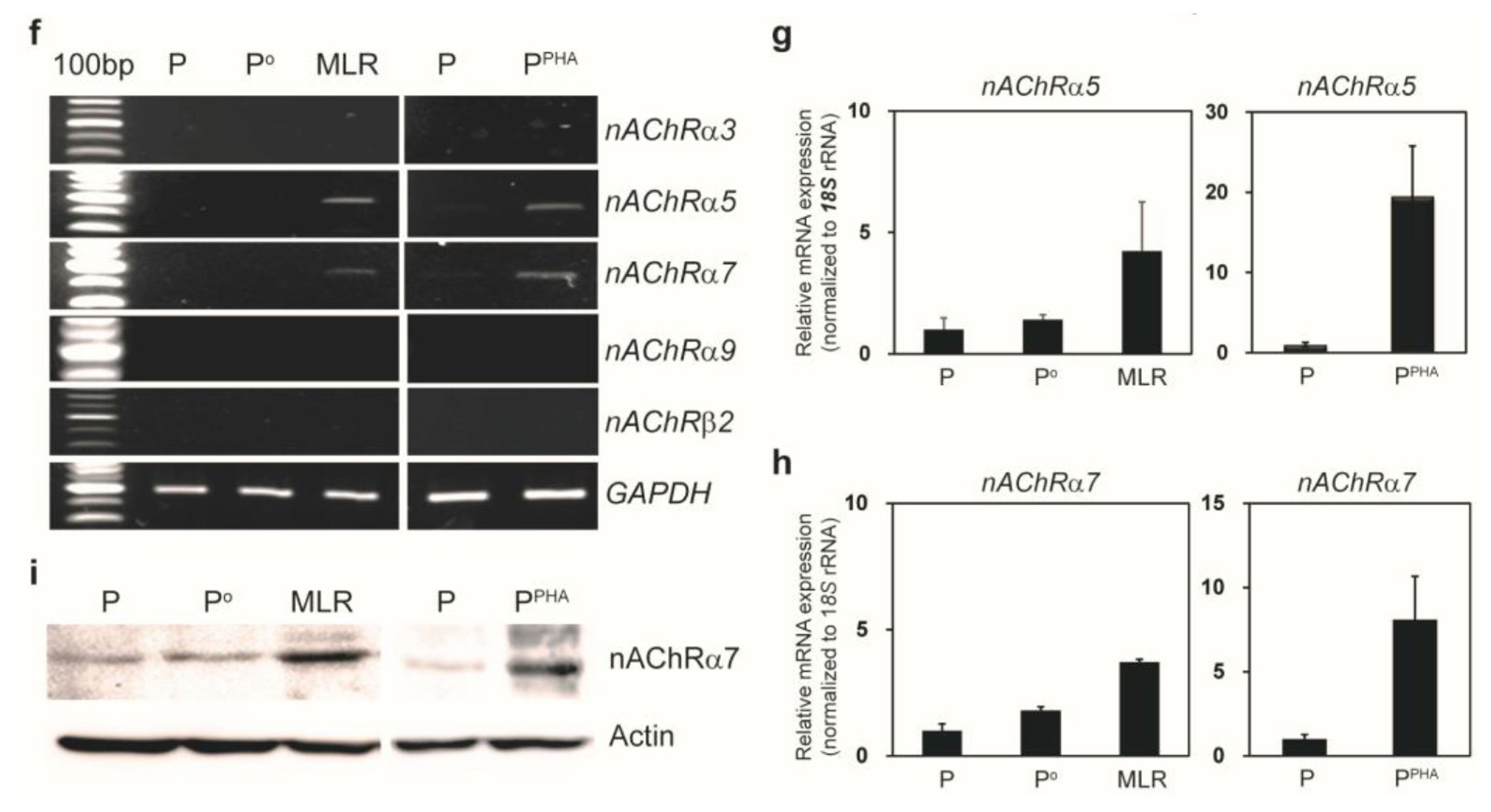
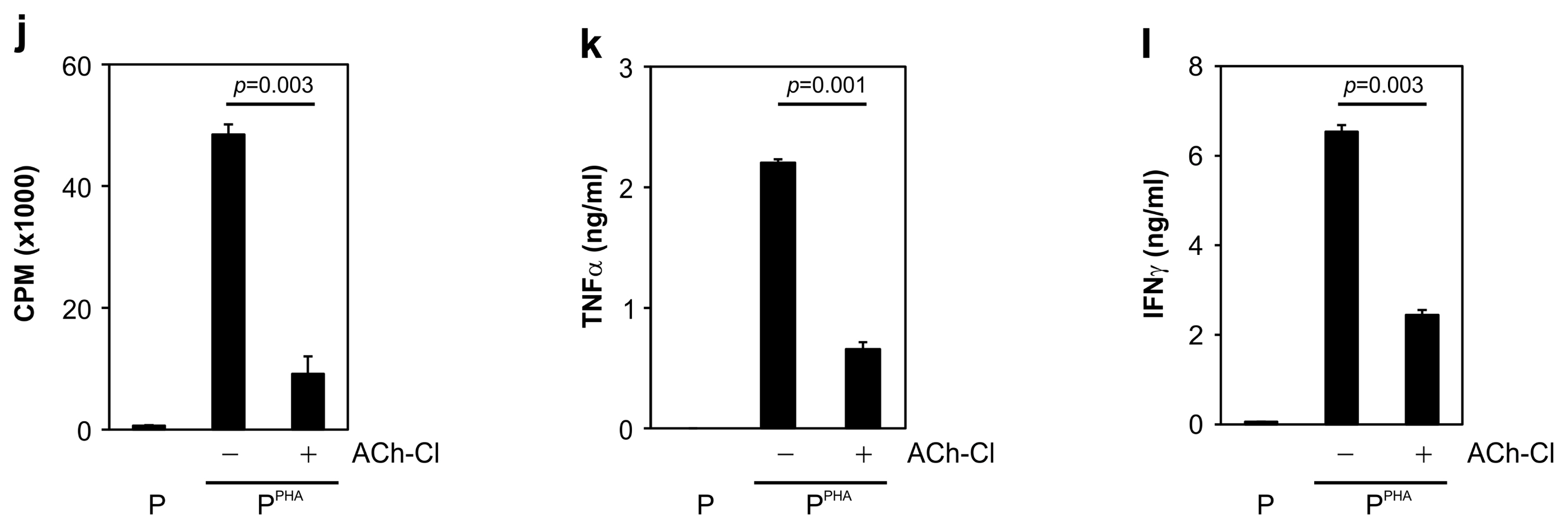
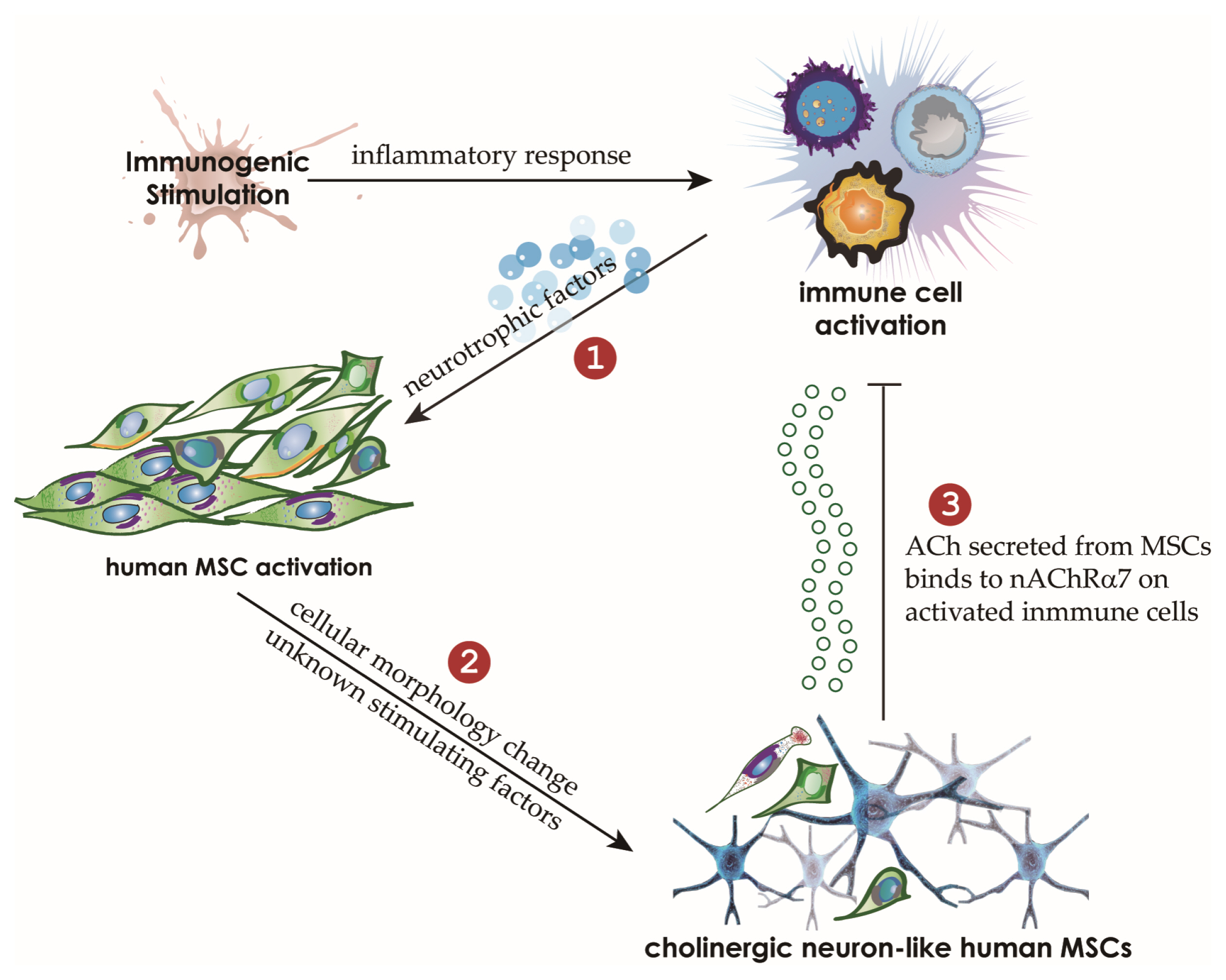
We cocultured MSCs with peripheral blood mononuclear cells for a mixed lymphocyte reaction or stimulated them by phytohemagglutinin. Morphological changes of MSCs and secretion of acetylcholine (ACh) from MSCs were measured. The effects of an ACh antagonist and ACh agonist on lymphocyte proliferation and proinflammatory-cytokine production were determined. The inflammatory milieu created by immune-cell activation caused MSCs to adopt a neuronlike phenotype and induced them to release ACh. Additionally, nicotinic acetylcholine receptors (nAChRs) were upregulated in activated peripheral blood mononuclear cells. We observed that ACh bound to nAChR on activated immune cells and led to the inhibition of lymphocyte proliferation and of proinflammatory-cytokine production. MSC-mediated immunosuppression through ACh activity was reversed by an ACh antagonist called α-bungarotoxin, and lymphocyte proliferation was inhibited by an ACh agonist, ACh chloride.
CONCLUSIONS
Our findings point to a novel immunomodulatory mechanism in which ACh secreted by MSCs under inflammatory conditions might modulate immune cells. This study may provide a novel method for the treatment of autoimmune diseases by means of MSCs.
MeSH Terms
Figure
Cited by 1 articles
-
Perivascular Stem Cells Suppress Inflammasome Activation during Inflammatory Responses in Macrophages
Jeeyoung Kim, Woo Jin Kim, Kwon-Soo Ha, Eun-Taek Han, Won Sun Park, Se-Ran Yang, Seok-Ho Hong
Int J Stem Cells. 2019;12(3):419-429. doi: 10.15283/ijsc19115.
Reference
-
References
1. Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008; 9:129–136. DOI: 10.1038/ni1560. PMID: 18204427. PMCID: PMC2696344.
Article2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article3. Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010; 21:1045–1056. DOI: 10.1089/hum.2010.115. PMID: 20565251. PMCID: PMC4823383.
Article4. Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, Tredici G. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther. 2011; 6:82–92. DOI: 10.2174/157488811795495486. PMID: 21190538.
Article5. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 18:683–692. DOI: 10.1089/scd.2008.0253. PMID: 19099374. PMCID: PMC3190292.
Article6. De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012; 12:574–591. DOI: 10.2174/156652412800619950. PMID: 22515979.
Article7. Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009; 11:377–391. DOI: 10.1080/14653240903080367. PMID: 19568970.
Article8. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008; 371:1579–1586. DOI: 10.1016/S0140-6736(08)60690-X. PMID: 18468541.
Article9. Parekkadan B, Upadhyay R, Dunham J, Iwamoto Y, Mizoguchi E, Mizoguchi A, Weissleder R, Yarmush ML. Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes. Gastroenterology. 2011; 140:966–975. DOI: 10.1053/j.gastro.2010.10.013. PMCID: PMC3033974.
Article10. Jung KH, Song SU, Yi T, Jeon MS, Hong SW, Zheng HM, Lee HS, Choi MJ, Lee DH, Hong SS. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011; 140:998–1008. DOI: 10.1053/j.gastro.2010.11.047. PMID: 21130088.
Article11. Na K, Yoo HS, Zhang YX, Choi MS, Lee K, Yi TG, Song SU, Jeon MS. Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell Death Dis. 2014; 5:e1345. DOI: 10.1038/cddis.2014.299. PMID: 25032868. PMCID: PMC4123091.
Article12. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013; 13:856–867. DOI: 10.2174/1566524011313050016. PMID: 23642066.
Article13. Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012; 67:1–8. DOI: 10.1111/j.1600-0897.2011.01069.x. PMID: 21951555.
Article14. Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006; 6:318–328. DOI: 10.1038/nri1810. PMID: 16557263. PMCID: PMC1783839.
Article15. Lukewich MK, Rogers RC, Lomax AE. Divergent neuroendocrine responses to localized and systemic inflammation. Semin Immunol. 2014; 26:402–408. DOI: 10.1016/j.smim.2014.01.004. PMID: 24486057. PMCID: PMC4128895.
Article16. Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol. 2014; 26:357–368. DOI: 10.1016/j.smim.2014.01.003. PMID: 24486056. PMCID: PMC4116469.
Article17. Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012; 30:313–335. DOI: 10.1146/annurev-immunol-020711-075015. PMID: 22224768. PMCID: PMC4533843.
Article18. Kawashima K, Fujii T. The lymphocytic cholinergic system and its biological function. Life Sci. 2003; 72:2101–2109. DOI: 10.1016/S0024-3205(03)00068-7. PMID: 12628464.
Article19. Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, Kawashima K. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett. 1999; 266:17–20. DOI: 10.1016/S0304-3940(99)00259-1. PMID: 10336173.
Article20. Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003; 9:125–134. DOI: 10.1007/BF03402177. PMID: 14571320. PMCID: PMC1430829.
Article21. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003; 421:384–388. DOI: 10.1038/nature01339. PMID: 12508119.
Article22. Fujii T, Takada-Takatori Y, Kawashima K. Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008; 106:186–192. DOI: 10.1254/jphs.FM0070109. PMID: 18285654.
Article23. Fujii YX, Fujigaya H, Moriwaki Y, Misawa H, Kasahara T, Grando SA, Kawashima K. Enhanced serum antigen-specific IgG1 and proinflammatory cytokine production in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. J Neuroimmunol. 2007; 189:69–74. DOI: 10.1016/j.jneuroim.2007.07.003. PMID: 17675251.
Article24. Hoogduijn MJ, Cheng A, Genever PG. Functional nicotinic and muscarinic receptors on mesenchymal stem cells. Stem Cells Dev. 2009; 18:103–112. DOI: 10.1089/scd.2008.0032. PMID: 18393628.
Article25. Hendrickson ML, Rao AJ, Demerdash ON, Kalil RE. Expression of nestin by neural cells in the adult rat and human brain. PLoS One. 2011; 6:e18535. DOI: 10.1371/journal.pone.0018535. PMID: 21490921. PMCID: PMC3072400.
Article26. Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003; 4:299–309. DOI: 10.1038/nrn1078. PMID: 12671646.
Article27. Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D’Elios MM, De Carli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997; 100:408–414. DOI: 10.1016/S0091-6749(97)70256-2. PMID: 9314355.
Article28. Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000; 86:29–48. DOI: 10.1016/S0163-7258(99)00071-6. PMID: 10760545.
Article29. Grando SA, Kawashima K, Wessler I. Introduction: the non-neuronal cholinergic system in humans. Life Sci. 2003; 72:2009–2012. DOI: 10.1016/S0024-3205(03)00063-8. PMID: 12628450.
Article30. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000; 405:458–462. DOI: 10.1038/35013070. PMID: 10839541.
Article31. Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007; 80:2314–2319. DOI: 10.1016/j.lfs.2007.02.036. PMID: 17383684.
Article32. Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey KJ, Al-Abed Y, Metz CN. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005; 201:1113–1123. DOI: 10.1084/jem.20040463. PMID: 15809354. PMCID: PMC2213139.
Article33. Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T. Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology. 2006; 50:540–547. DOI: 10.1016/j.neuropharm.2005.10.013. PMID: 16336980.
Article34. Shen JX, Yakel JL. Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin. 2009; 30:673–680. DOI: 10.1038/aps.2009.64. PMID: 19448647. PMCID: PMC4002362.
Article35. Fauchais AL, Boumediene A, Lalloue F, Gondran G, Loustaud-Ratti V, Vidal E, Jauberteau MO. Brain-derived neurotrophic factor and nerve growth factor correlate with T-cell activation in primary Sjogren’s syndrome. Scand J Rheumatol. 2009; 38:50–57. DOI: 10.1080/03009740802378832. PMID: 18830907.
Article36. Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993; 90:10984–10988. DOI: 10.1073/pnas.90.23.10984. PMID: 7902578. PMCID: PMC47906.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Immunomodulatory Effect of Epidermal Growth Factor Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells on Atopic Dermatitis
- Bone marrow-derived stem cells contribute to regeneration of the endometrium