Int J Stem Cells.
2019 Jul;12(2):265-278. 10.15283/ijsc18136.
Characterization and Differentiation of Circulating Blood Mesenchymal Stem Cells and the Role of Phosphatidylinositol 3-Kinase in Modulating the Adhesion
- Affiliations
-
- 1Dental Research Institute, Seoul National University, Brain Korea 21, Seoul, Korea.
- 2Dental Research Institute and Prosthodontics, Seoul National University Dental Hospital, School of Dentistry, Seoul National University, Seoul, Korea. ksy0617@snu.ac.kr
- 3Department of Dental Regenerative Biotechnology, School of Dentistry, Seoul National University, Seoul, Korea. jcho@snu.ac.kr
- KMID: 2465897
- DOI: http://doi.org/10.15283/ijsc18136
Abstract
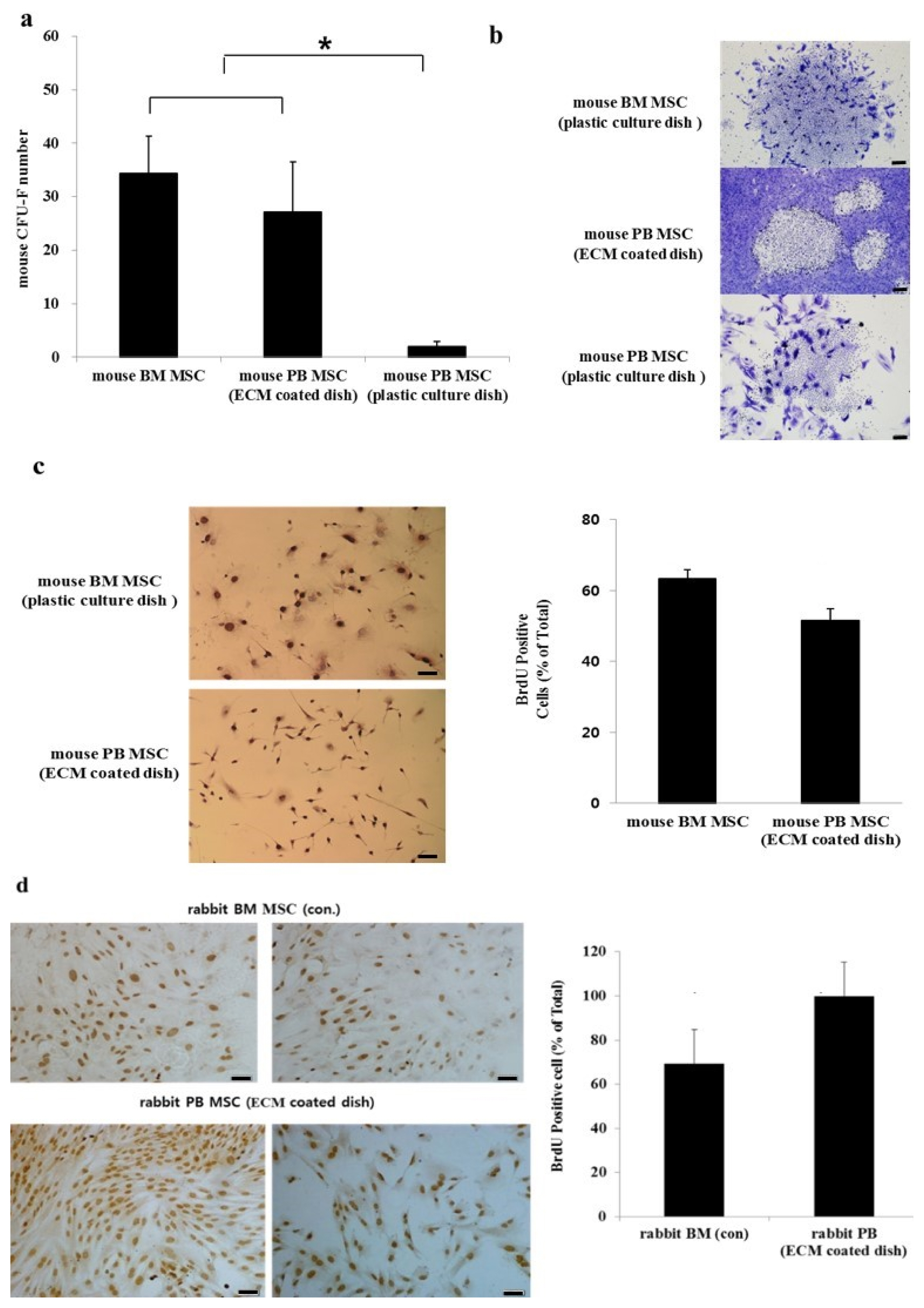
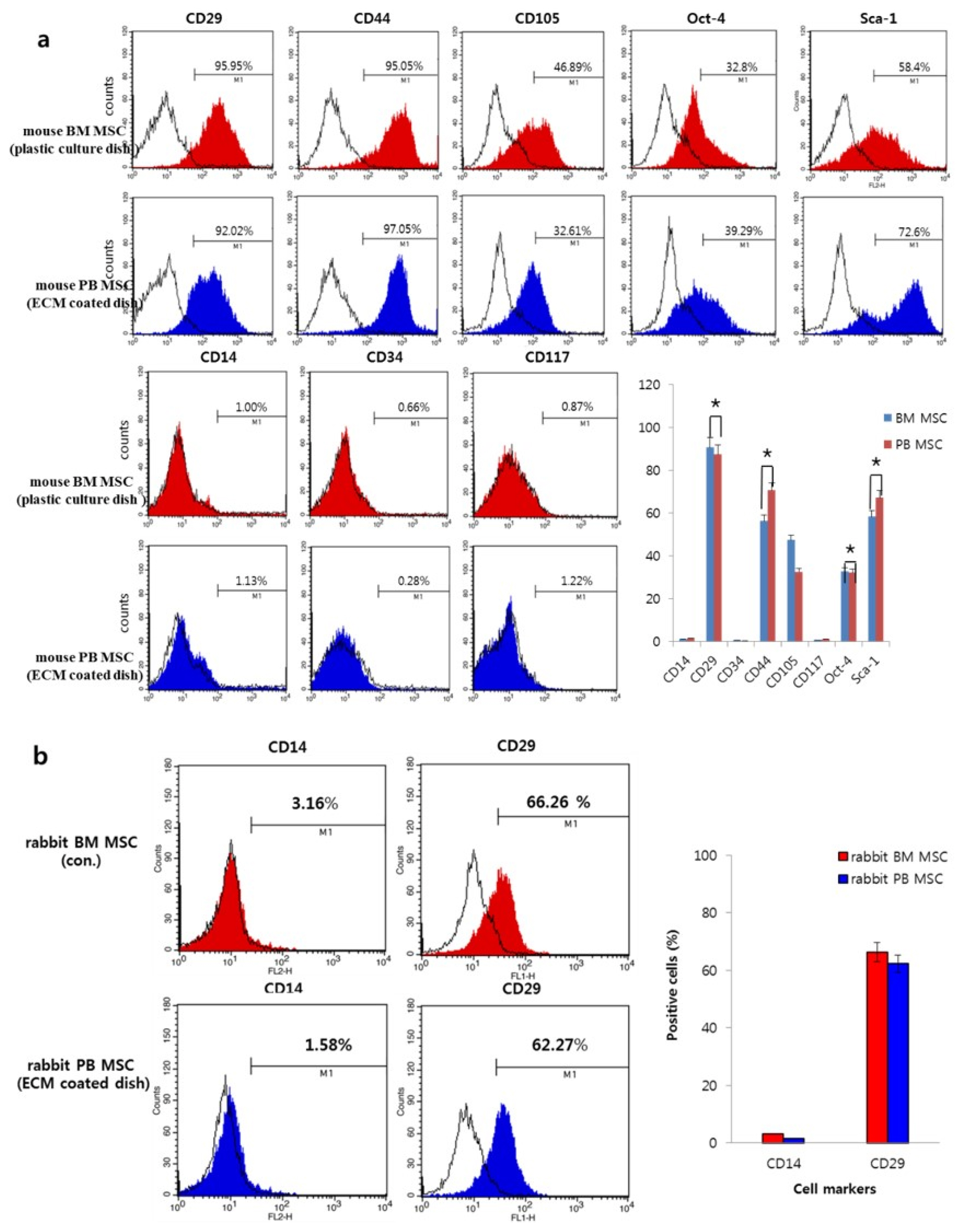
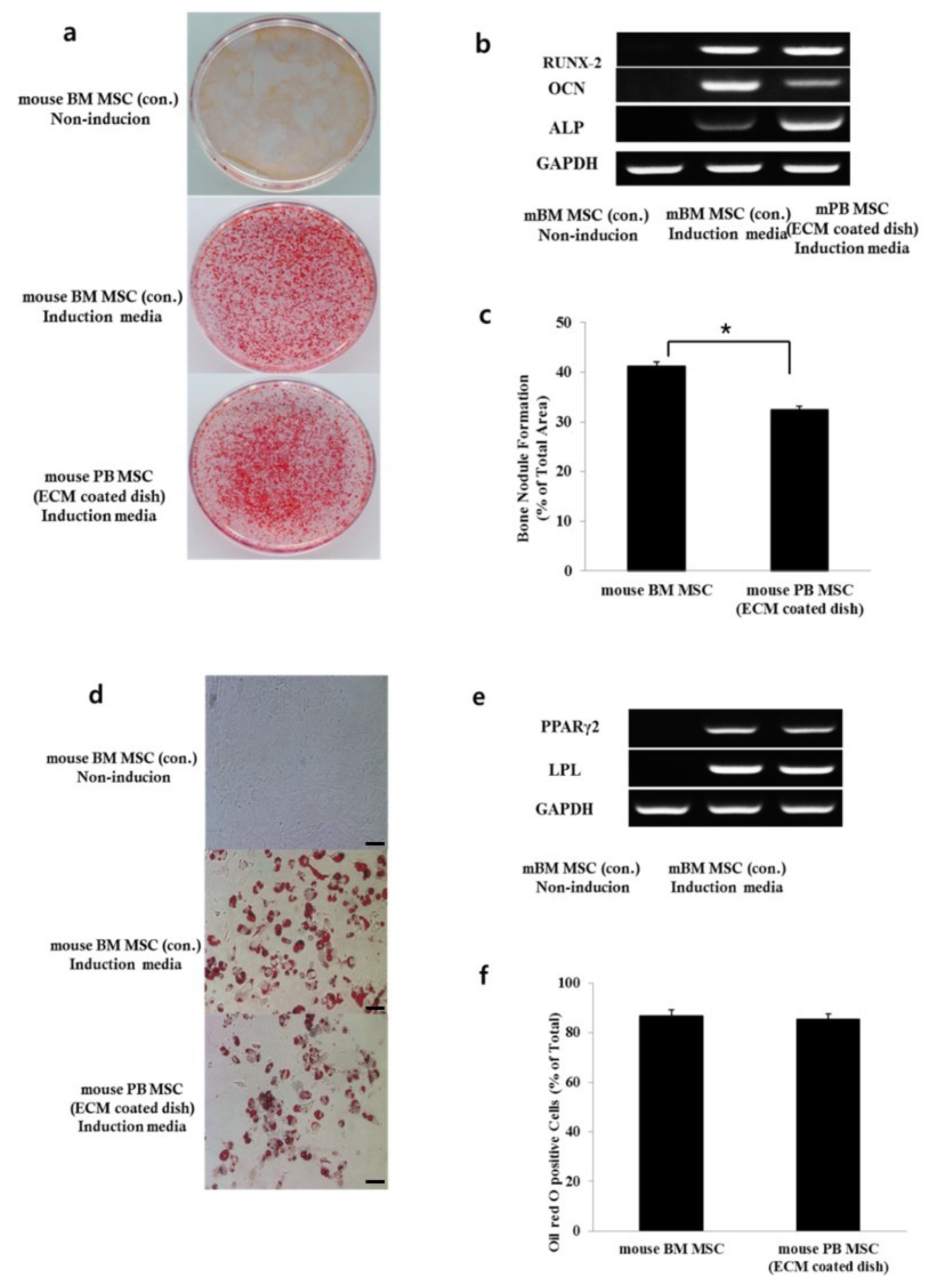
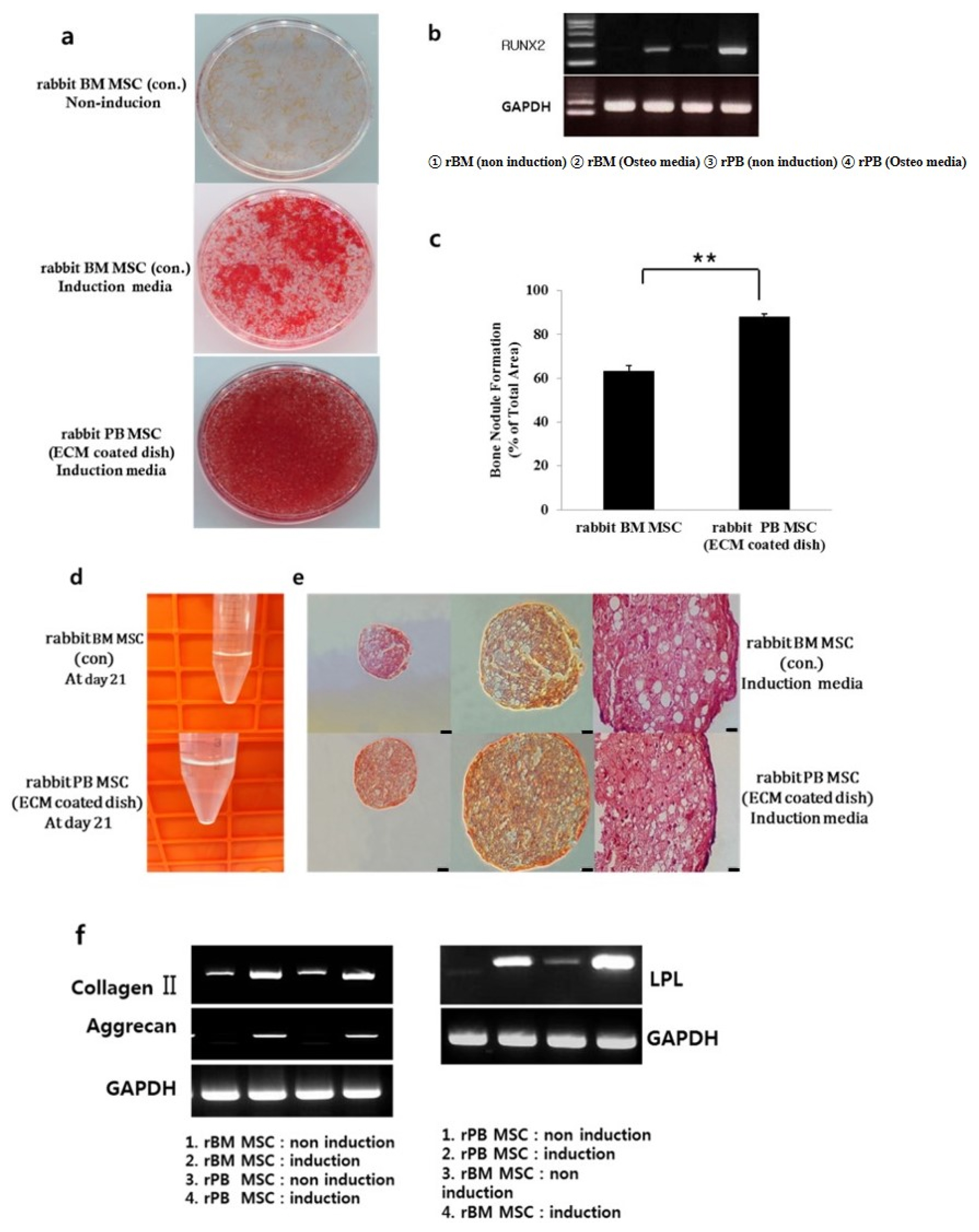
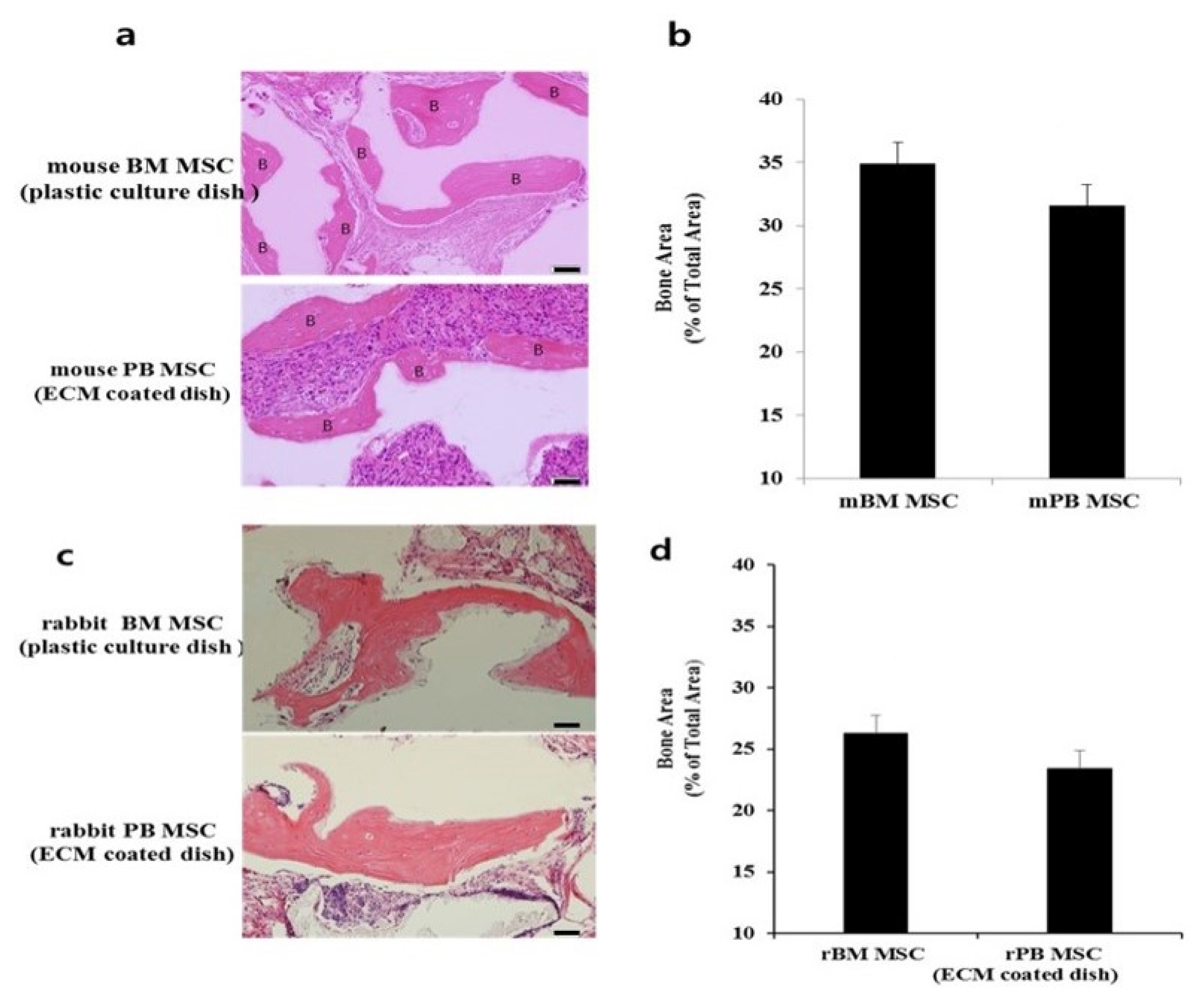
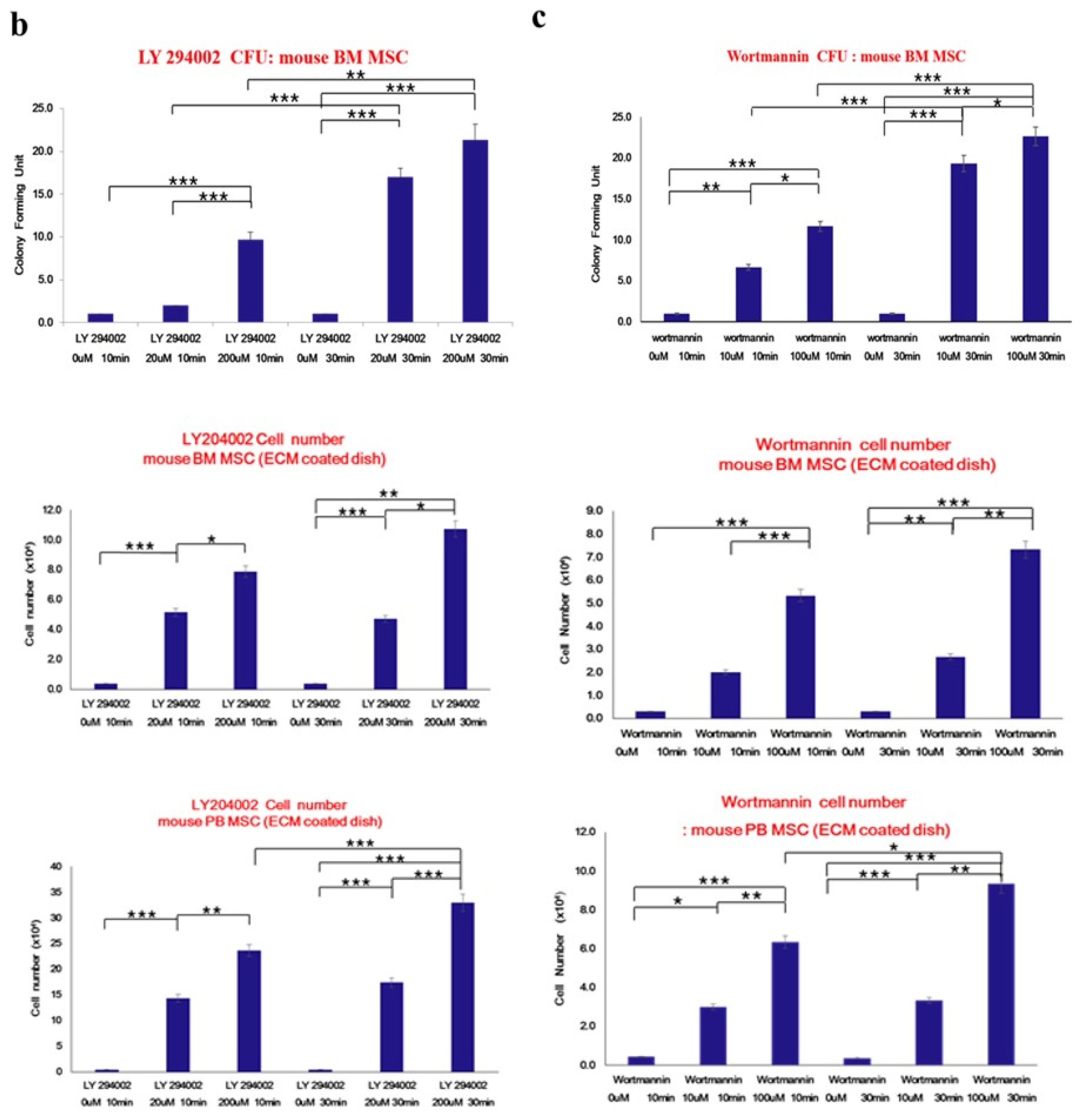
- Bone marrow mesenchymal stem cells (BM MSCs) can differentiate into multi-lineage tissues. However, obtaining BM MSCs by aspiration is difficult and can be painful; therefore peripheral blood (PB) MSCs might provide an easier alternative for clinical applications. Here, we show that circulating PB MSCs proliferate as efficiently as BM MSCs in the presence of extracellular matrix (ECM) and that differentiation potential into osteoblast in vitro and in vivo. Both BM MSCs and PB MSCs developed into new bone when subcutaneously transplanted into immune-compromised mice using hydroxyapatite/tricalcium phosphate as a carrier. Furthermore, LY294002 and Wortmannin blocked mesenchymal stem cell attachment in a dose-dependent manner, suggesting a role of phosphatidylinositol 3-kinase in MSC attachment. Our data showed that the growth of PB MSCs could be regulated by interaction with the ECM and that these cells could differentiate into osteoblasts, suggesting their potential for clinical applications.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015; 35:pii: e00191. DOI: 10.1042/BSR20150025. PMID: 25797907. PMCID: 4413017.
Article2. Perlin JR, Sporrij A, Zon LI. Blood on the tracks: hematopoietic stem cell-endothelial cell interactions in homing and engraftment. J Mol Med (Berl). 2017; 95:809–819. DOI: 10.1007/s00109-017-1559-8. PMID: 28702683. PMCID: PMC5558790.
Article3. Xie C, Yang Z, Suo Y, Chen Q, Wei D, Weng X, Gu Z, Wei X. Systemically infused mesenchymal stem cells show different homing profiles in healthy and tumor mouse models. Stem Cells Transl Med. 2017; 6:1120–1131. DOI: 10.1002/sctm.16-0204. PMID: 28205428. PMCID: 5442841.
Article4. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007; 25:2739–2749. DOI: 10.1634/stemcells.2007-0197. PMID: 17656645.
Article5. Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015; 17:11–22. DOI: 10.1016/j.stem.2015.06.007. PMID: 26140604.
Article6. Goldberg A, Mitchell K, Soans J, Kim L, Zaidi R. The use of mesenchymal stem cells for cartilage repair and regeneration: a systematic review. J Orthop Surg Res. 2017; 12:39. DOI: 10.1186/s13018-017-0534-y. PMID: 28279182. PMCID: 5345159.
Article7. Liotta F, Annunziato F, Castellani S, Boddi M, Alterini B, Castellini G, Mazzanti B, Cosmi L, Acquafresca M, Bartalesi F, Dilaghi B, Dorigo W, Graziani G, Bartolozzi B, Bellandi G, Carli G, Bartoloni A, Fargion A, Fassio F, Fontanari P, Landini G, Lucente EAM, Michelagnoli S, Orsi Battaglini C, Panigada G, Pigozzi C, Querci V, Santarlasci V, Parronchi P, Troisi N, Baggiore C, Romagnani P, Mannucci E, Saccardi R, Pratesi C, Gensini G, Romagnani S, Maggi E. Therapeutic efficacy of autologous non-mobilized enriched circulating endothelial progenitors in patients with critical limb ischemia - the SCELTA trial. Circ J. 2018; 82:1688–1698. DOI: 10.1253/circj.CJ-17-0720. PMID: 29576595.
Article8. Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016; 17:230. DOI: 10.1186/s12891-016-1085-9. PMID: 27229856. PMCID: 4880954.
Article9. Zheng RC, Park YK, Cho JJ, Kim SK, Heo SJ, Koak JY, Lee JH. Bone regeneration at dental implant sites with suspended stem cells. J Dent Res. 2014; 93:1005–1013. DOI: 10.1177/0022034514548706. PMID: 25183420. PMCID: 4293714.
Article10. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003; 21:105–110. DOI: 10.1634/stemcells.21-1-105. PMID: 12529557.
Article11. Fahy N, Alini M, Stoddart MJ. Mechanical stimulation of mesenchymal stem cells: implications for cartilage tissue engineering. J Orthop Res. 2018; 36:52–63. DOI: 10.1002/jor.23670. PMID: 28763118.
Article12. Fadini GP, Ciciliot S, Albiero M. Concise review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells. 2017; 35:106–116. DOI: 10.1002/stem.2445. PMID: 27401837.
Article13. Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp Hematol. 2015; 43:498–513. DOI: 10.1016/j.exphem.2015.04.011. PMID: 25970610.
Article14. Zheng RC, Park YK, Kim SK, Cho J, Heo SJ, Koak JY, Lee SJ, Park JM, Lee JH, Kim JH. Bone regeneration of blood-derived stem cells within dental implants. J Dent Res. 2015; 94:1318–1325. DOI: 10.1177/0022034515590368. PMID: 26078421.
Article15. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003; 121:368–374. DOI: 10.1046/j.1365-2141.2003.04284.x. PMID: 12694261.
Article16. Hassan G, Kasem I, Soukkarieh C, Aljamali M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells: using explant method and umbilical cord blood serum. Int J Stem Cells. 2017; 10:184–192. DOI: 10.15283/ijsc17028. PMID: 28844128. PMCID: 5741200.
Article17. Ward PS, Thompson CB. Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol. 2012; 4:a006783. DOI: 10.1101/cshperspect.a006783. PMID: 22687276. PMCID: 3385956.
Article18. Scanlon V, Soung do Y, Adapala NS, Morgan E, Hansen MF, Drissi H, Sanjay A. Role of Cbl-PI3K interaction during skeletal remodeling in a murine model of bone repair. PLoS One. 2015; 10:e0138194. DOI: 10.1371/journal.pone.0138194. PMID: 26393915. PMCID: 4578922.
Article19. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017; 170:605–635. DOI: 10.1016/j.cell.2017.07.029. PMID: 28802037. PMCID: 5726441.
Article20. Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004; 22:313–323. DOI: 10.1634/stemcells.22-3-313. PMID: 15153608.
Article21. Hidalgo-Bastida LA, Cartmell SH. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng Part B Rev. 2010; 16:405–412. DOI: 10.1089/ten.teb.2009.0714. PMID: 20163206.
Article22. Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005; 23:1105–1112. DOI: 10.1634/stemcells.2004-0330. PMID: 15955825.
Article23. He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007; 25:69–77. DOI: 10.1634/stemcells.2006-0335. PMID: 16973831.
Article24. Khosla S, Westendorf JJ, Mödder UI. Concise review: insights from normal bone remodeling and stem cell-based therapies for bone repair. Stem Cells. 2010; 28:2124–2128. DOI: 10.1002/stem.546. PMID: 20960512. PMCID: 3125598.
Article25. Wu G, Pan M, Wang X, Wen J, Cao S, Li Z, Li Y, Qian C, Liu Z, Wu W, Zhu L, Guo J. Osteogenesis of peripheral blood mesenchymal stem cells in self assembling peptide nanofiber for healing critical size calvarial bony defect. Sci Rep. 2015; 5:16681. DOI: 10.1038/srep16681. PMID: 26568114. PMCID: 4645224.
Article26. Lakkakorpi PT, Wesolowski G, Zimolo Z, Rodan GA, Rodan SB. Phosphatidylinositol 3-kinase association with the osteoclast cytoskeleton, and its involvement in osteoclast attachment and spreading. Exp Cell Res. 1997; 237:296–306. DOI: 10.1006/excr.1997.3797. PMID: 9434625.
Article27. Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015; 210:503–515. DOI: 10.1083/jcb.201501025. PMID: 26216901. PMCID: 4523609.
Article28. Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007; 22:1943–1956. DOI: 10.1359/jbmr.070725. PMID: 17680726.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- The Role of Histone Acetylation in Mesenchymal Stem Cell Differentiation
- In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood