Int J Stem Cells.
2019 Nov;12(3):484-496. 10.15283/ijsc19090.
Maintenance of hPSCs under Xeno-Free and Chemically Defined Culture Conditions
- Affiliations
-
- 1Department of Biomedical Science, Graduate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea. ks66kim@hanyang.ac.kr
- 2Soonchunhyang Institute of Medi-bioscience, Soonchunhyang University, Cheonan, Korea.
- 31st Research Center, Axceso Biopharma Co., Ltd., Yongin, Korea.
- 4Department of Internal Medicine, School of Medicine, Kangwon National University, Chuncheon, Korea. shhong@kangwon.ac.kr
- 5College of Medicine, Hanyang University, Seoul, Korea.
- KMID: 2465889
- DOI: http://doi.org/10.15283/ijsc19090
Abstract
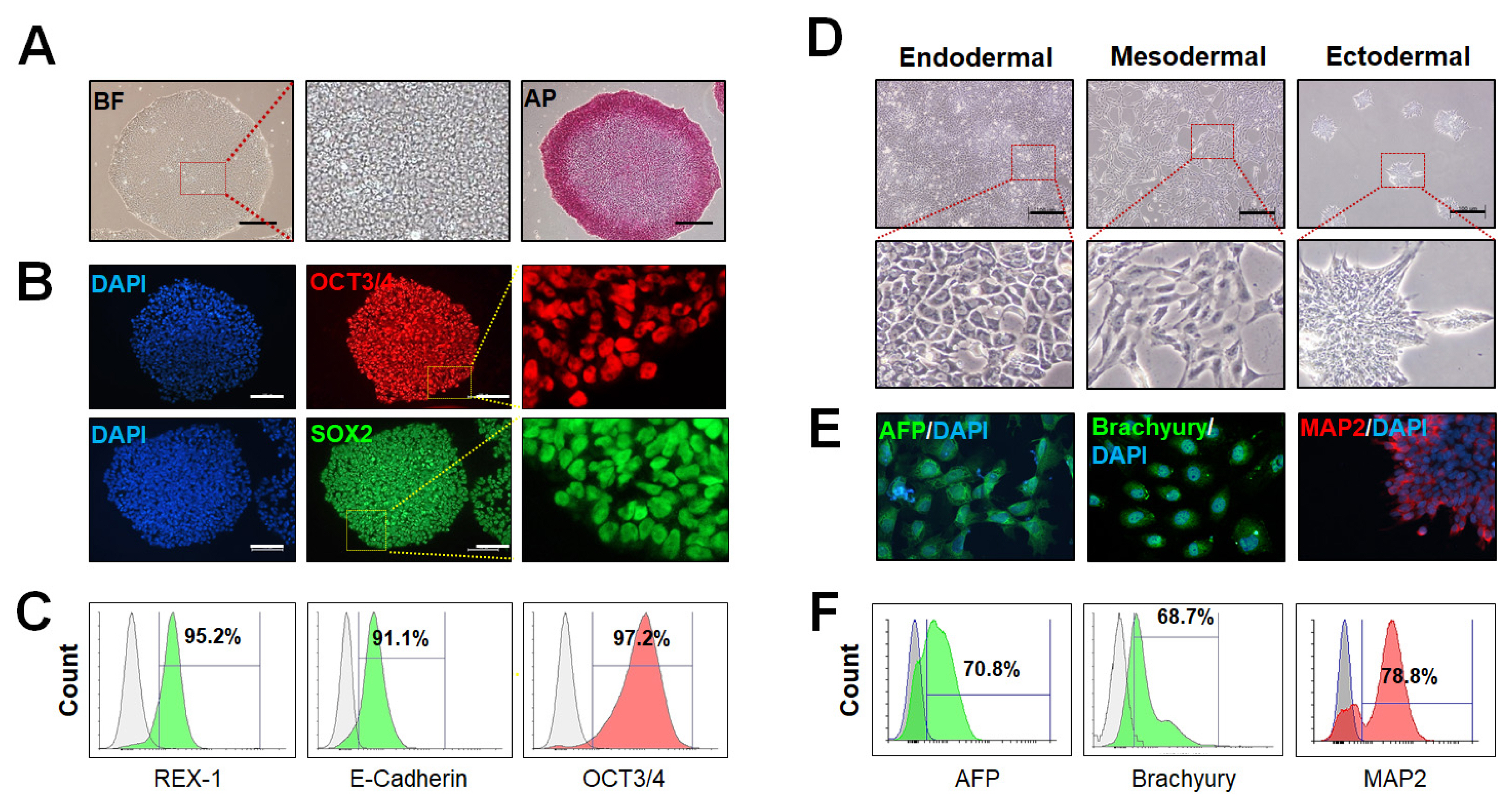
- Previously, the majority of human embryonic stem cells and human induced pluripotent stem cells have been derived on feeder layers and chemically undefined medium. Those media components related to feeder cells, or animal products, often greatly affect the consistency of the cell culture. There are clear advantages of a defined, xeno-free, and feeder-free culture system for human pluripotent stem cells (hPSCs) cultures, since consistency in the formulations prevents lot-to-lot variability. Eliminating all non-human components reduces health risks for downstream applications, and those environments reduce potential immunological reactions from stem cells. Therefore, development of feeder-free hPSCs culture systems has been an important focus of hPSCs research. Recently, researchers have established a variety of culture systems in a defined combination, xeno-free matrix and medium that supports the growth and differentiation of hPSCs. Here we described detailed hPSCs culture methods under feeder-free and chemically defined conditions using vitronetin and TeSR-E8 medium including supplement bioactive lysophospholipid for promoting hPSCs proliferation and maintaining stemness.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article2. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318:1917–1920. DOI: 10.1126/science.1151526. PMID: 18029452.
Article3. Dakhore S, Nayer B, Hasegawa K. Human pluripotent stem cell culture: current status, challenges, and advancement. Stem Cells Int. 2018; 2018:7396905. DOI: 10.1155/2018/7396905. PMID: 30595701. PMCID: PMC6282144.
Article4. Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001; 19:971–974. DOI: 10.1038/nbt1001-971. PMID: 11581665.
Article5. Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002; 20:933–936. DOI: 10.1038/nbt726. PMID: 12161760.
Article6. Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003; 21:546–556. DOI: 10.1634/stemcells.21-5-546. PMID: 12968109.
Article7. Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006; 24:185–187. DOI: 10.1038/nbt1177. PMID: 16388305.
Article8. Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011; 8:424–429. DOI: 10.1038/nmeth.1593. PMID: 21478862. PMCID: PMC3084903.
Article9. Yasuda SY, Ikeda T, Shahsavarani H, Yoshida N, Nayer B, Hino M, Sharma NV, Suemori H, Hasegawa K. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nat Biomed Eng. 2018; 2:173–182. DOI: 10.1038/s41551-018-0200-7. PMID: 31015717.
Article10. Phadnis SM, Loewke NO, Dimov IK, Pai S, Amwake CE, Solgaard O, Baer ™, Chen B, Pera RAR. Dynamic and social behaviors of human pluripotent stem cells. Sci Rep. 2015; 5:14209. DOI: 10.1038/srep14209. PMID: 26381699. PMCID: PMC4585647.
Article11. Jacobs K, Zambelli F, Mertzanidou A, Smolders I, Geens M, Nguyen HT, Barbé L, Sermon K, Spits C. Higher-density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Reports. 2016; 6:330–341. DOI: 10.1016/j.stemcr.2016.01.015. PMID: 26923824. PMCID: PMC4788786.
Article12. Hongisto H, Vuoristo S, Mikhailova A, Suuronen R, Virtanen I, Otonkoski T, Skottman H. Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture. Stem Cell Res. 2012; 8:97–108. DOI: 10.1016/j.scr.2011.08.005. PMID: 22099024.
Article13. Inzunza J, Gertow K, Strömberg MA, Matilainen E, Blennow E, Skottman H, Wolbank S, Ahrlund-Richter L, Hovatta O. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005; 23:544–549. DOI: 10.1634/stemcells.2004-0201. PMID: 15790775.
Article14. Viswanathan P, Gaskell T, Moens N, Culley OJ, Hansen D, Gervasio MK, Yeap YJ, Danovi D. Human pluripotent stem cells on artificial microenvironments: a high content perspective. Front Pharmacol. 2014; 5:150. DOI: 10.3389/fphar.2014.00150. PMID: 25071572. PMCID: PMC4078252.
Article15. Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013; 24:830–833. DOI: 10.1016/j.copbio.2013.04.011. PMID: 23726156. PMCID: PMC3773047.
Article16. Kefalides NA. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973; 6:63–104. DOI: 10.1016/B978-0-12-363706-2.50008-8. PMID: 4198817.
Article17. Timpl R, Rohde H, Risteli L, Ott U, Robey PG, Martin GR. Laminin. Methods Enzymol. 1982; 82(Pt A):831–838. DOI: 10.1016/0076-6879(82)82104-6. PMID: 7078458.18. Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979; 254:9933–9937. PMID: 114518.
Article19. Chung AE, Jaffe R, Freeman IL, Vergnes JP, Braginski JE, Carlin B. Properties of a basement membrane-related glycoprotein synthesized in culture by a mouse embryonal carcinoma-derived cell line. Cell. 1979; 16:277–287. DOI: 10.1016/0092-8674(79)90005-9. PMID: 88263.
Article20. Hassell JR, Robey PG, Barrach HJ, Wilczek J, Rennard SI, Martin GR. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980; 77:4494–4498. DOI: 10.1073/pnas.77.8.4494. PMID: 6449008. PMCID: PMC349870.
Article21. Carlin B, Jaffe R, Bender B, Chung AE. Entactin, a novel basal lamina-associated sulfated glycoprotein. J Biol Chem. 1981; 256:5209–5214. PMID: 6262321.
Article22. Cooper AR, MacQueen HA. Subunits of laminin are differentially synthesized in mouse eggs and early embryos. Dev Biol. 1983; 96:467–471. DOI: 10.1016/0012-1606(83)90183-5. PMID: 6403399.
Article23. Ekblom P, Vestweber D, Kemler R. Cell-matrix interactions and cell adhesion development. Annu Rev Cell Biol. 1986; 2:27–47. DOI: 10.1146/annurev.cb.02.110186.000331. PMID: 3548769.24. Hierck BP, Thorsteinsdóttir S, Niessen CM, Freund E, Iperen LV, Feyen A, Hogervorst F, Poelmann RE, Mummery CL, Sonnenberg A. Variants of the alpha 6 beta 1 laminin receptor in early murine development: distribution, molecular cloning and chromosomal localization of the mouse integrin alpha 6 subunit. Cell Adhes Commun. 1993; 1:33–53. DOI: 10.3109/15419069309095680. PMID: 8081870.
Article25. Cooper HM, Tamura RN, Quaranta V. The major laminin receptor of mouse embryonic stem cells is a novel isoform of the alpha 6 beta 1 integrin. J Cell Biol. 1991; 115:843–850. DOI: 10.1083/jcb.115.3.843. PMID: 1833411. PMCID: PMC2289180.
Article26. Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982; 21:6188–6193. DOI: 10.1021/bi00267a025. PMID: 6217835.
Article27. Kleinman HK, McGarvey ML, Hassell JR, Martin GR. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochemistry. 1983; 22:4969–4974. DOI: 10.1021/bi00290a014. PMID: 6227336.
Article28. Mackay AR, Gomez DE, Cottam DW, Rees RC, Nason AM, Thorgeirsson UP. Identification of the 72-kDa (MMP-2) and 92-kDa (MMP-9) gelatinase/type IV collagenase in preparations of laminin and Matrigel. Biotechniques. 1993; 15:1048–1051. PMID: 8292337.29. Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992; 202:1–8. DOI: 10.1016/0014-4827(92)90397-Q. PMID: 1511725.
Article30. Borg TK. It’s the matrix! ECM, proteases, and cancer. Am J Pathol. 2004; 164:1141–1142. DOI: 10.1016/S0002-9440(10)63201-4. PMID: 15039202. PMCID: PMC1615333.31. Li J, Bardy J, Yap LY, Chen A, Nurcombe V, Cool SM, Oh SK, Birch WR. Impact of vitronectin concentration and surface properties on the stable propagation of human embryonic stem cells. Biointerphases. 2010; 5:FA132–FA142. DOI: 10.1116/1.3525804. PMID: 21171706.
Article32. Doolittle JM, Gomez SM. Structural similarity-based predictions of protein interactions between HIV-1 and Homo sapiens. Virol J. 2010; 7:82. DOI: 10.1186/1743-422X-7-82. PMID: 20426868. PMCID: PMC2877021.
Article33. Juliano RL, Varner JA. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993; 5:812–818. DOI: 10.1016/0955-0674(93)90030-T. PMID: 8240825.
Article34. Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, Lebrin F, Kats P, Hochstenbach R, Passier R, Sonnenberg A, Mummery CL. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008; 26:2257–2265. DOI: 10.1634/stemcells.2008-0291. PMID: 18599809.
Article35. Pulido D, Hussain SA, Hohenester E. Crystal structure of the heterotrimeric integrin-binding region of laminin-111. Structure. 2017; 25:530–535. DOI: 10.1016/j.str.2017.01.002. PMID: 28132784. PMCID: PMC5343747.
Article36. Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014; 14:13–26. DOI: 10.1016/j.stem.2013.12.005. PMID: 24388173. PMCID: PMC3915741.
Article37. Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum- free medium devoid of animal-derived products. Biotechnol Bioeng. 2005; 91:688–698. DOI: 10.1002/bit.20536. PMID: 15971228.
Article38. Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not -332, -111, or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008; 26:2800–2809. DOI: 10.1634/stemcells.2007-0389. PMID: 18757303.
Article39. Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol. 2010; 28:611–615. DOI: 10.1038/nbt.1620. PMID: 20512123.
Article40. Rodin S, Antonsson L, Hovatta O, Tryggvason K. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521-based matrices under xeno-free and chemically defined conditions. Nat Protoc. 2014; 9:2354–2368. DOI: 10.1038/nprot.2014.159. PMID: 25211513.
Article41. Villa-Diaz LG, Kim JK, Laperle A, Palecek SP, Krebsbach PH. Inhibition of focal adhesion kinase signaling by integrin α6β1 supports human pluripotent stem cell self-renewal. Stem Cells. 2016; 34:1753–1764. DOI: 10.1002/stem.2349. PMID: 26930028.
Article42. Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling and termination at lipid phosphate receptors. Biochim Biophys Acta. 2002; 1582:121–131. DOI: 10.1016/S1388-1981(02)00146-4. PMID: 12069819.
Article43. Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002; 277:25851–28584. DOI: 10.1074/jbc.R200007200. PMID: 12011102.
Article44. Draper JS, Fox V. Human embryonic stem cells: multi-lineage differentiation and mechanisms of self-renewal. Arch Med Res. 2003; 34:558–564. DOI: 10.1016/j.arcmed.2003.08.006.
Article45. Inniss K, Moore H. Mediation of apoptosis and proliferation of human embryonic stem cells by sphingosine-1-phosphate. Stem Cells Dev. 2006; 15:789–796. DOI: 10.1089/scd.2006.15.789. PMID: 17253942.
Article46. Kim MK, Park KS, Lee H, Kim YD, Yun J, Bae YS. Phytosphingosine-1-phosphate stimulates chemotactic migration of L2071 mouse fibroblasts via pertussis toxin-sensitive G-proteins. Exp Mol Med. 2007; 39:185–194. DOI: 10.1038/emm.2007.21. PMID: 17464180.
Article47. Pata MO, Hannun YA, Ng CK. Plant sphingolipids: decoding the enigma of the Sphinx. New Phytol. 2010; 185:611–630. DOI: 10.1111/j.1469-8137.2009.03123.x. PMID: 20028469. PMCID: PMC2848707.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Engineering Biomaterials for Feeder-Free Maintenance of Human Pluripotent Stem Cells
- Adaptation of Human Testicular Niche Cells for Pluripotent Stem Cell and Testis Development Research
- Optimal Xeno-free Culture Condition for Clinical Grade Stem Cells from Human Exfoliated Deciduous Teeth
- Influences of Xeno-Free Media on Mesenchymal Stem Cell Expansion for Clinical Application
- Clean-Up Human Embryonic Stem Cell Lines Using Humanized Culture Condition