Korean Circ J.
2020 Jan;50(1):52-68. 10.4070/kcj.2019.0173.
Comparison of First-Line Dual Combination Treatments in Hypertension: Real-World Evidence from Multinational Heterogeneous Cohorts
- Affiliations
-
- 1Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea. veritas@ajou.ac.kr
- 2MediBloc Inc., Seoul, Korea.
- 3Janssen Research and Development, Titusville, NJ, USA.
- 4Department of Biomathematics, University of California, Los Angeles, CA, USA.
- 5Department of Biostatistics, University of California, Los Angeles, CA, USA.
- 6Department of Human Genetics, University of California, Los Angeles, CA, USA.
- 7Department of Biomedical Informatics, Columbia University Medical Center, New York, NY, USA.
- 8Medical Informatics Services, NewYork-Presbyterian Hospital, New York, NY, USA.
- 9Department of Biomedical Sciences, Ajou University Graduate School of Medicine, Suwon, Korea.
- 10Division of Cardiology, Severance Cardiovascular Hospital and Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul, Korea. shpark0530@yuhs.ac
- KMID: 2465664
- DOI: http://doi.org/10.4070/kcj.2019.0173
Abstract
- BACKGROUND AND OBJECTIVES
2018 ESC/ESH Hypertension guideline recommends 2-drug combination as initial anti-hypertensive therapy. However, real-world evidence for effectiveness of recommended regimens remains limited. We aimed to compare the effectiveness of first-line anti-hypertensive treatment combining 2 out of the following classes: angiotensin-converting enzyme (ACE) inhibitors/angiotensin-receptor blocker (A), calcium channel blocker (C), and thiazide-type diuretics (D).
METHODS
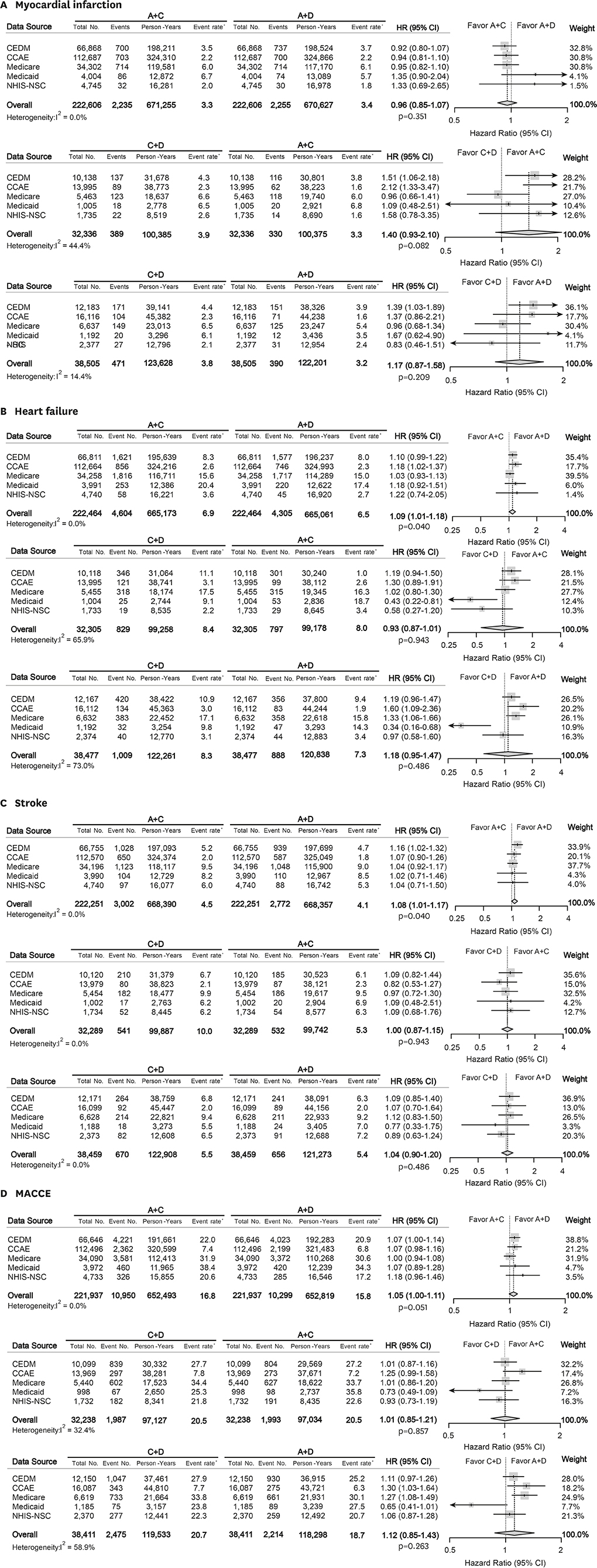
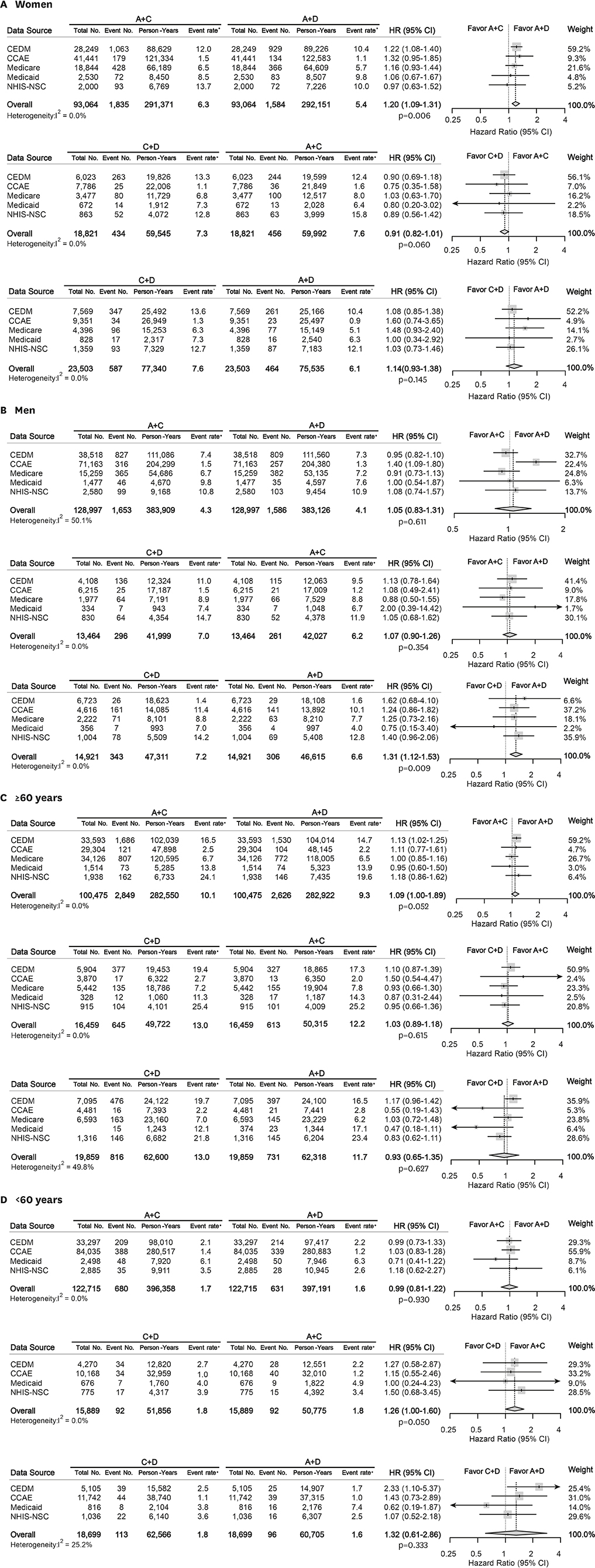
Treatment-naïve hypertensive adults without cardiovascular disease (CVD) who initiated dual anti-hypertensive medications were identified in 5 databases from US and Korea. The patients were matched for each comparison set by large-scale propensity score matching. Primary endpoint was all-cause mortality. Myocardial infarction, heart failure, stroke, and major adverse cardiac and cerebrovascular events as a composite outcome comprised the secondary measure.
RESULTS
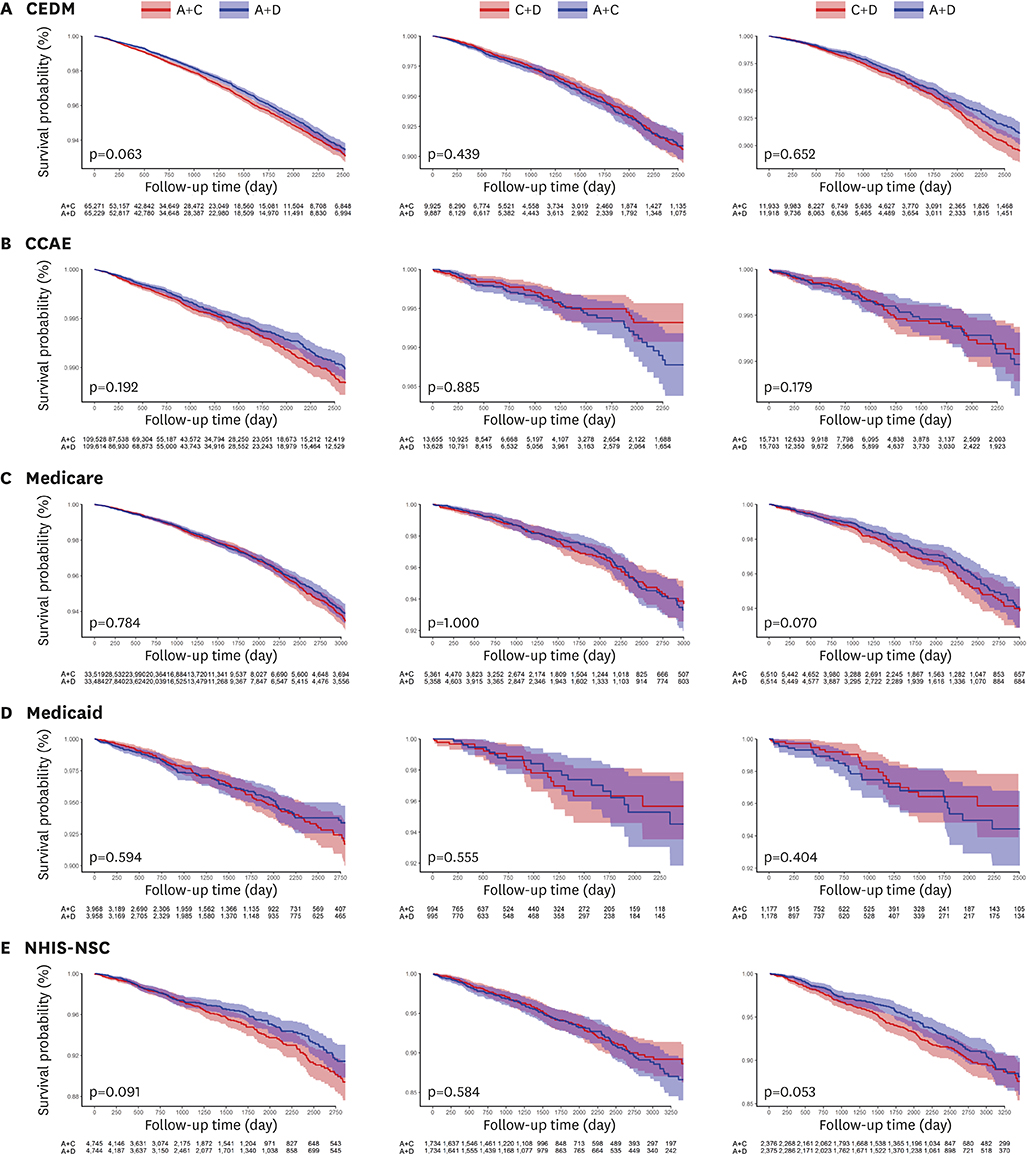
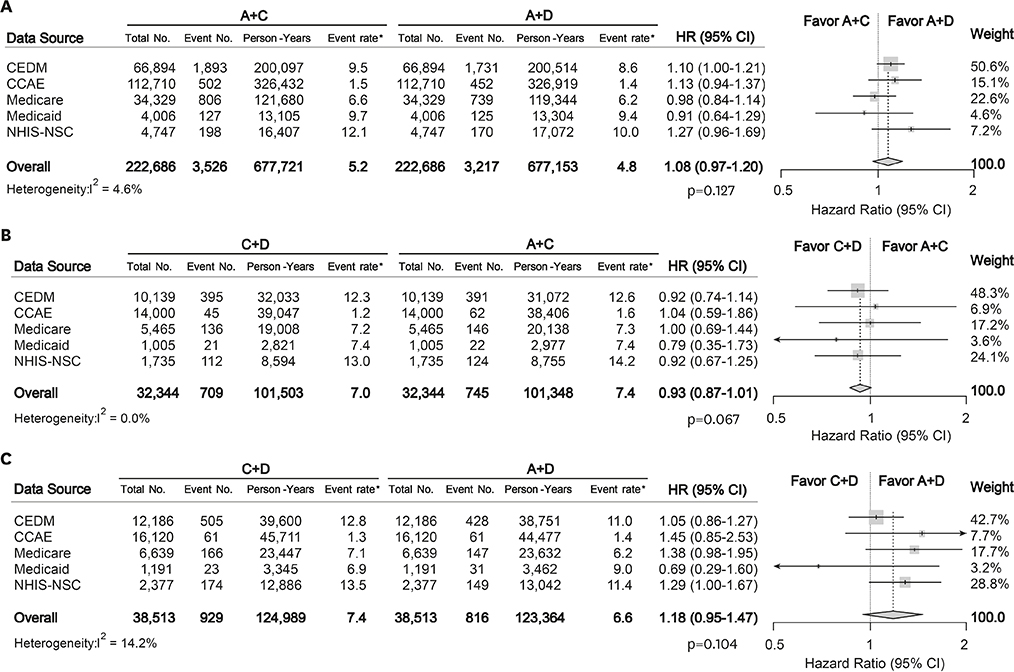
A total of 987,983 patients met the eligibility criteria. After matching, 222,686, 32,344, and 38,513 patients were allocated to A+C vs. A+D, C+D vs. A+C, and C+D vs. A+D comparison, respectively. There was no significant difference in the mortality during total of 1,806,077 person-years: A+C vs. A+D (hazard ratio [HR], 1.08; 95% confidence interval [CI], 0.97−1.20; p=0.127), C+D vs. A+C (HR, 0.93; 95% CI, 0.87−1.01; p=0.067), and C+D vs. A+D (HR, 1.18; 95% CI, 0.95−1.47; p=0.104). A+C was associated with a slightly higher risk of heart failure (HR, 1.09; 95% CI, 1.01−1.18; p=0.040) and stroke (HR, 1.08; 95% CI, 1.01−1.17; p=0.040) than A+D.
CONCLUSIONS
There was no significant difference in mortality among A+C, A+D, and C+D combination treatment in patients without previous CVD. This finding was consistent across multi-national heterogeneous cohorts in real-world practice.
Keyword
MeSH Terms
-
Adult
Angiotensin Receptor Antagonists
Antihypertensive Agents
Calcium Channel Blockers
Calcium Channels
Cardiovascular Diseases
Cohort Studies*
Diuretics
Heart Failure
Humans
Hypertension*
Korea
Mortality
Myocardial Infarction
Propensity Score
Stroke
Angiotensin Receptor Antagonists
Antihypertensive Agents
Calcium Channel Blockers
Calcium Channels
Diuretics
Figure
Cited by 1 articles
-
Big Challenge in Big Data Research: Continual Dispute on Big Data Analysis
Hae-Young Lee
Korean Circ J. 2020;50(1):69-71. doi: 10.4070/kcj.2019.0349.
Reference
-
1. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388:1659–1724.2. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004; 363:2022–2031.
Article3. Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002; 4:393–404.4. Basile JN, Bloch MJ. Analysis of recent papers in hypertension. Initial combination therapy provides more prompt blood pressure control and reduces cardiovascular events but remains underutilized. J Clin Hypertens (Greenwich). 2013; 15:523–525.
Article5. Gradman AH, Parisé H, Lefebvre P, Falvey H, Lafeuille MH, Duh MS. Initial combination therapy reduces the risk of cardiovascular events in hypertensive patients: a matched cohort study. Hypertension. 2013; 61:309–318.6. Egan BM, Bandyopadhyay D, Shaftman SR, Wagner CS, Zhao Y, Yu-Isenberg KS. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012; 59:1124–1131.
Article7. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39:3021–3104.8. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008; 359:2417–2428.
Article9. Ogihara T, Saruta T, Rakugi H, et al. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: a randomized, controlled trial. J Hypertens. 2014; 32:2054–2063.10. Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003; 290:2805–2816.
Article11. Weber MA, Jamerson K, Bakris GL, et al. Effects of body size and hypertension treatments on cardiovascular event rates: subanalysis of the ACCOMPLISH randomised controlled trial. Lancet. 2013; 381:537–545.
Article12. Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015; 216:574–578.13. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15.
Article14. Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018; 47:2005–2014.
Article15. Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013; 23:10.1145/2414416.2414791.
Article16. Suissa S. Effectiveness of inhaled corticosteroids in chronic obstructive pulmonary disease: immortal time bias in observational studies. Am J Respir Crit Care Med. 2003; 168:49–53.17. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010; 21:383–388.18. Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017; 66:72–81.
Article19. Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci U S A. 2018; 115:2571–2577.
Article20. Lee H, Park S, Kim HC. Temporal and geospatial trends of hypertension management in Korea: a nationwide study 2002–2016. Korean Circ J. 2019; 49:514–527.
Article21. Matsuzaki M, Ogihara T, Umemoto S, et al. Prevention of cardiovascular events with calcium channel blocker-based combination therapies in patients with hypertension: a randomized controlled trial. J Hypertens. 2011; 29:1649–1659.22. Chi C, Tai C, Bai B, et al. Angiotensin system blockade combined with calcium channel blockers is superior to other combinations in cardiovascular protection with similar blood pressure reduction: a meta-analysis in 20,451 hypertensive patients. J Clin Hypertens (Greenwich). 2016; 18:801–808.
Article23. Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003; 289:2534–2544.24. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens. 2015; 33:1321–1341.25. Wassertheil-Smoller S, Psaty B, Greenland P, et al. Association between cardiovascular outcomes and antihypertensive drug treatment in older women. JAMA. 2004; 292:2849–2859.
Article26. Turnbull F, Woodward M, Neal B, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008; 29:2669–2680.
Article27. Corrao G, Zambon A, Parodi A, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens. 2008; 26:819–824.
Article28. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010; 55:399–407.29. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018; 71:1474–1482.30. Lin DY, Zeng D. On the relative efficiency of using summary statistics versus individual-level data in meta-analysis. Biometrika. 2010; 97:321–332.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pragmatic Clinical Trials for Real-World Evidence: Concept and Implementation
- Real-World Evidence of Trastuzumab, Pertuzumab, and Docetaxel Combination as a First-Line Treatment for Korean Patients with HER2-Positive Metastatic Breast Cancer
- Medicine in the Fourth Industrial Revolution: What Should We Prepare?
- Antihypertensive drug combinations
- Case Analysis in Drug Approval by FDA/EMA Using Real-World Data/Real-World Evidence