Korean Circ J.
2020 Jan;50(1):22-34. 10.4070/kcj.2019.0097.
SYNTAX Score and SYNTAX Score II Can Predict the Clinical Outcomes of Patients with Left Main and/or 3-Vessel Disease Undergoing Percutaneous Coronary Intervention in the Contemporary Cobalt-Chromium Everolimus-Eluting Stent Era
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine and Cardiovascular Centre, Seoul National University Hospital, Seoul, Korea. hpcrates@gmail.com
- KMID: 2465660
- DOI: http://doi.org/10.4070/kcj.2019.0097
Abstract
- BACKGROUND AND OBJECTIVES
The impact of SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery score (SS) and SS II in patients who receive percutaneous coronary intervention with second-generation everolimus-eluting stents (EES) has not been fully validated.
METHODS
The SS, SS II were calculated in 1,248 patients with left main and/or 3-vessel disease treated with EES. Patient-oriented composite endpoint (POCE; all-cause death, any myocardial infarction (MI), any revascularization) and target lesion failure (TLF: cardiac death, target-vessel MI, target lesion revascularization) were analyzed.
RESULTS
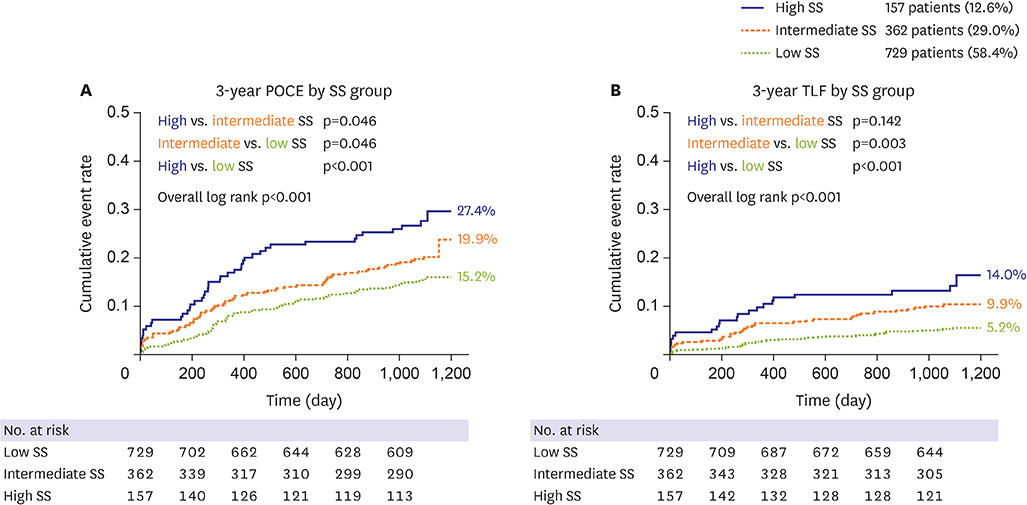
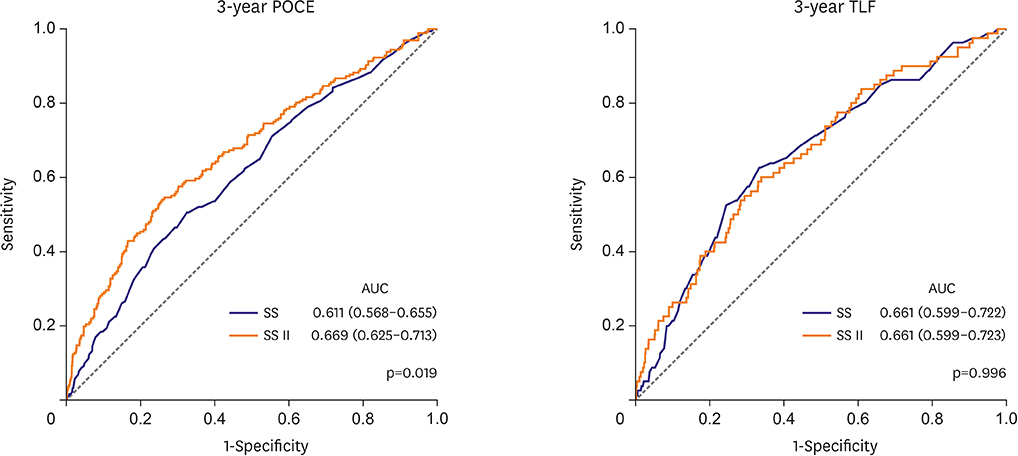
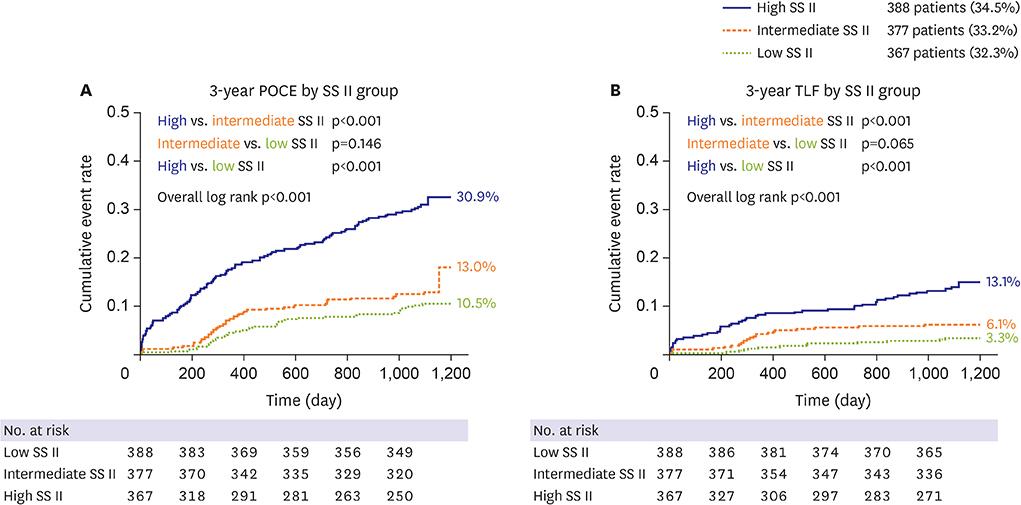
The mean SS was 21.1±9.6. Three-year POCE increased according to the SS group (15.2% vs. 19.9% vs. 27.4% for low (≤22), intermediate (≥23, ≤32), high (≥33) SS groups, p<0.001). By multivariate Cox proportional hazard analysis, SS group was an independent predictor of 3-year POCE (hazard ratio, 1.324; 95% confidence interval, 1.095-1.601; p=0.004). The receiver operating characteristic curves revealed that the SS II was superior to the SS for 3-year POCE prediction (area under the curve [AUC]: 0.611 vs. 0.669 for SS vs. SS II, p=0.019), but not for 3-year TLF (AUC: 0.631 vs. 0.660 for SS vs. SS II, p=0.996). In subgroup analysis, SS II was superior to SS in patients with cardiovascular clinical risk factors, and in those presenting as stable angina.
CONCLUSIONS
The usefulness of SS and SS II was still valid in patients with left main and/or 3-vessel disease. SS II was superior to SS for the prediction of patient-oriented outcomes, but not for lesion-oriented outcomes. TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT00698607 ClinicalTrials.gov Identifier: NCT01605721
MeSH Terms
Figure
Cited by 1 articles
-
Risk Stratification by SYNTAX Score Systems in Current Percutaneous Revascularization Era
Choongki Kim
Korean Circ J. 2020;50(1):35-37. doi: 10.4070/kcj.2019.0348.
Reference
-
1. Kolh P, Windecker S. ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J. 2014; 35:3235–3236.2. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014; 64:e139–e228.3. Morice MC, Serruys PW, Kappetein AP, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation. 2014; 129:2388–2394.
Article4. Head SJ, Davierwala PM, Serruys PW, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J. 2014; 35:2821–2830.
Article5. Kang SH, Park KW, Kang DY, et al. Biodegradable-polymer drug-eluting stents vs. bare metal stents vs. durable-polymer drug-eluting stents: a systematic review and Bayesian approach network meta-analysis. Eur Heart J. 2014; 35:1147–1158.
Article6. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013; 347:f6625.
Article7. Navarese EP, Tandjung K, Claessen B, et al. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ. 2013; 347:f6530.
Article8. Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014; 63:299–307.9. Kang J, Park KW, Han JK, et al. Usefulness of the baseline syntax score to predict 3-year outcome after complete revascularization by percutaneous coronary intervention. Am J Cardiol. 2016; 118:641–646.
Article10. Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013; 381:639–650.
Article11. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27:157–172.
Article12. Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009; 360:961–972.
Article13. Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013; 381:629–638.
Article14. Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011; 58:e123–e210.15. Park KW, Kang J, Kang SH, et al. Usefulness of the SYNTAX and clinical SYNTAX scores in predicting clinical outcome after unrestricted use of sirolimus- and everolimus-eluting stents. Circ J. 2013; 77:2912–2921.
Article16. Park KW, Kang J, Kang SH, et al. The impact of residual coronary lesions on clinical outcomes after percutaneous coronary intervention: residual SYNTAX score after percutaneous coronary intervention in patients from the Efficacy of Xience/Promus versus Cypher in rEducing Late Loss after stENTing (EXCELLENT) registry. Am Heart J. 2014; 167:384–392.e5.
Article17. Garg S, Sarno G, Garcia-Garcia HM, et al. A new tool for the risk stratification of patients with complex coronary artery disease: the clinical SYNTAX score. Circ Cardiovasc Interv. 2010; 3:317–326.
Article18. Nam CW, Mangiacapra F, Entjes R, et al. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011; 58:1211–1218.
Article19. Chieffo A, Meliga E, Latib A, et al. Drug-eluting stent for left main coronary artery disease. The DELTA registry: a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc Interv. 2012; 5:718–727.20. Xu B, Généreux P, Yang Y, et al. Validation and comparison of the long-term prognostic capability of the SYNTAX score-II among 1,528 consecutive patients who underwent left main percutaneous coronary intervention. JACC Cardiovasc Interv. 2014; 7:1128–1137.
Article21. He J, Zhao H, Yu X, et al. SYNTAX score-II predicts long-term mortality in patients who underwent left main percutaneous coronary intervention treated with second-generation drug-eluting stents. Int Heart J. 2017; 58:344–350.
Article22. Cavalcante R, Sotomi Y, Mancone M, et al. Impact of the SYNTAX scores I and II in patients with diabetes and multivessel coronary disease: a pooled analysis of patient level data from the SYNTAX, PRECOMBAT, and BEST trials. Eur Heart J. 2017; 38:1969–1977.
Article23. Song Y, Gao Z, Tang X, et al. Usefulness of the SYNTAX score II to validate 2-year outcomes in patients with complex coronary artery disease undergoing percutaneous coronary intervention: a large single-center study. Catheter Cardiovasc Interv. 2018; 92:40–47.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Implications of Early Exercise Treadmill Testing after Percutaneous Coronary Intervention in the Drug-eluting Stent Era
- Pentraxin 3 Is Highly Specific for Predicting Anatomical Complexity of Coronary Artery Stenosis as Determined by the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery Score
- Drug-Eluting Stent: Present and Future
- Is SYNTAX Score Predictive of Atrial Fibrillation after On-Pump Coronary Artery Bypass Graft Surgery?
- Dark Side of Drug-eluting Stent in Contemporary Percutaneous Coronary Intervention