Pediatr Infect Vaccine.
2015 Aug;22(2):106-112. 10.14776/piv.2015.22.2.106.
Molecular Diagnosis of Streptococcus pneumoniae in Middle Ear Fluids from Children with Otitis Media with Effusion
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. kaykim@ewha.ac.kr
- 3Center for Vaccine Evaluation and Study, Medical Research Institute, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2465255
- DOI: http://doi.org/10.14776/piv.2015.22.2.106
Abstract
- PURPOSE
The long-term administration of antibiotics interferes with bacterial culture in the middle ear fluids (MEFs) of young children with otitis media with effusion (OME). The purpose of this study is to determine whether molecular diagnostics can be used for rapid and direct detection of the bacterial pathogen in culture-negative MEFs.
METHODS
The specificity and sensitivity of both polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) to the lytA gene of Streptococcus pneumoniae were comparatively tested and then applied for pneumococcal detection in the clinical MEFs.
RESULTS
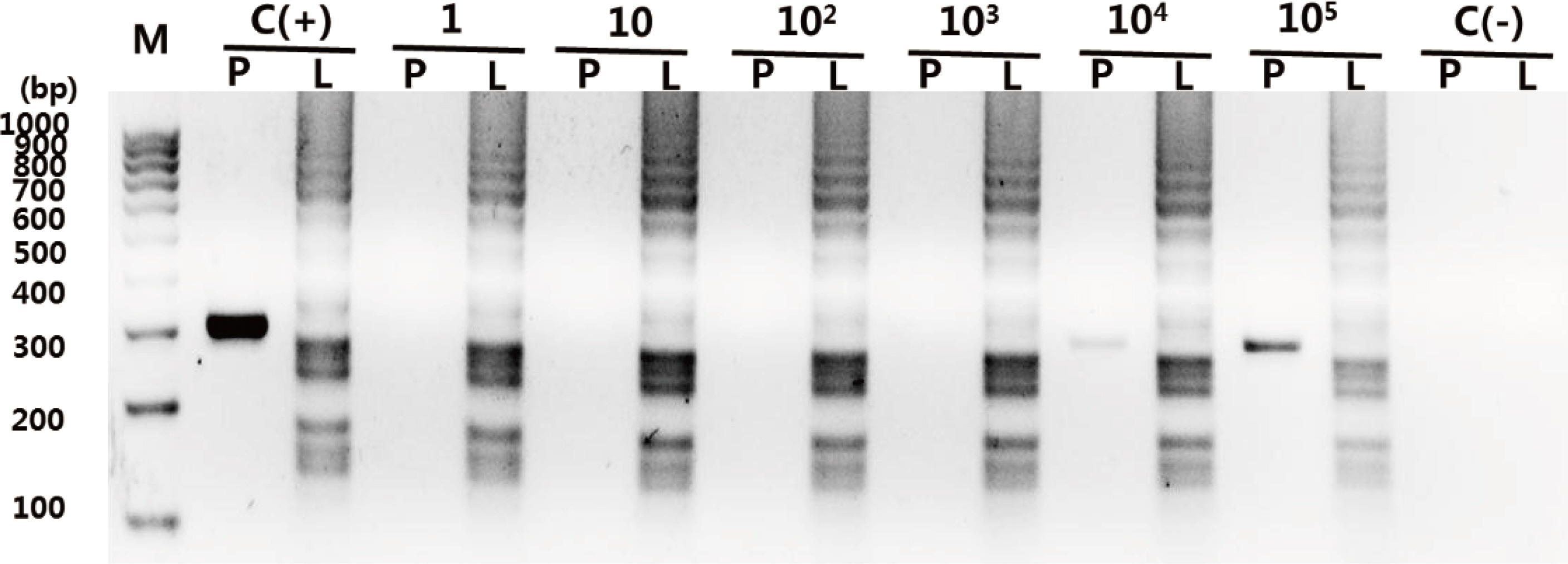
The detection limit of the PCR assay was approximately 10(4) colony forming units (CFU), whereas that of LAMP was less than 10 CFU for the detection of S. pneumoniae. Both PCR and LAMP did not amplify nucleic acid at over 10(6) CFU of H. influenzae or M. catarrhalis, both of which were irrelevant bacterial species. Of 22 culture-negative MEFs from children with OME, LAMP positivity was found in twelve MEFs (54.5%, 12/22), only three of which were PCR-positive (25%, 3/12). Our results showed that the ability of LAMP to detect pneumococcal DNA is over four times higher than that of PCR (P<0.01).
CONCLUSIONS
As a high-resolution tool able to detect nucleic acid levels equivalent to <10 CFU of S. pneumoniae in MEFs without any cross-reaction with other pathogens, lytA-specific LAMP may be applied for diagnosing pneumococcus infection in OME as well as evaluating the impact of a pneumococcal conjugate vaccine against OME.
Keyword
MeSH Terms
Figure
Reference
-
1. Howie VM, Ploussard JH, Lester RL, jr. Otitis media: a clinical and bacteriological correlation. Pediatrics. 1970; 45:29–35.2. Luotonen J, Herva E, Karma P, Timonen M, Leinonen M, Makela PH. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981; 13:177–83.
Article3. Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992; 11:S7–11.
Article4. Kilpi T, Herva E, Kaijalainen T, Syrjanen R, Takala AK. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr Infect Dis J. 2001; 20:654–62.
Article5. Qvarnberg Y, Holopainen E, Palva T. Aspiration cytology in acute otitis media. Acta Otolaryngol. 1984; 97:443–9.
Article6. Rosenfeld RM. An evidence—based approach to treating otitis media. Pediatr Clin North Am. 1996; 43:1165–81.
Article7. Rosenfeld RM, Schwartz SR, Pynnonen MA, Tunkel DE, Hussey HM, Fichera JS, et al. Clinical practice guideline: Tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013; 149:S1–35.8. Saleem M, Naz M, Waris A, Muneer B, Khurshid R. Screening of pneumococcal pneumonia by amplification of pneumolysin gene in children Visiting hospitals in lahore, pakistan. Iranian J Pediatr. 2012; 22:524–30.9. McAvin JC, Reilly PA, Roudabush RM, Barnes Wj, Salmen A, Iackson GW, et al. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using realtime fluorescence PCR. J Clin Microbiol. 2001; 39:3446–51.10. Morrison KE, Lake D, Crook J, Carlene GM, Ades E, Facklam R, et al. Confirmation of pspA in all 90 serotypes of Streptococcus pneumoniae by PCR and potential of this assay for identification and diagnosis. J Clin Microbiol. 2000; 38:434–7.11. Rintamaki S, Saukkoriipi A, Salo P, Takala A, Leinonen M. Detection of Streptococcus pneumoniae DNA by using polymerase chain reaction and microwell hybridization with Europium—labelled probes. J Clin Microbiol. 2002; 50:313–8.12. Kim DVV, Kilgore PE, Kim Ej, Kim SA, Anh DD, Dong BQ, et al. The enhanced pneumococcal LAMP assay: a clinical tool for the diagnosis of meningitis due to Streptococcus pneumoniae. PLoS One. 2012; 7:e42954.
Article13. Hoppe JE, Grieshaber , Hofler W. Colonization of Nigerian neonates with group B streptococci and its rapid detection. Infection. 1986; 14:74–8.
Article14. Prattes J, Koidl C, Eigl S, Krause R, Hoenigl M. Bronchoalveolar lavage fluid sample pretreatment with Sputasol (®) significantly reduces galactomannan levels. J Infect. 2015; 70:541–3.15. Nagai K, Shibasaki Y, Hasegawa K, Davies TA, Jacobs MR, Ubukata K, et al. Evaluation of PCR primers to screen for Streptococcus pneumoniae isolates and beta—lactam resistance, and to detect common macrolide resistance determinants. J Antimicrob Chemother. 2001; 48:915–8.
Article16. Black S, Shinefield HR, Fireman B, Lewis E, Ray P, Hansen JR, et al. Eflicacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000; 19:187–95.17. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Eng J Med. 2001; 344:403–9.
Article18. Berman S. Otitis media in children. N Eng J Med. 1995; 332:1560–5.
Article19. Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001; 293:498–506.20. Park JH, Kim KH, Andrade AL, Briles DE, McDaniel LS, Nahm MH. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. mBio. 2012; 3:e00035–12.
Article21. Approved lists of bacterial names. Med J Aust. 1980; 2:3–4.22. Ankerst J, Christensen P, Kjellen L, Kronvall G. A rountine diagnostic test for IgA and IgM antibodies to rubella Virus: absorption of IgG with Staphylococcus aureus. J Infect Dis. 1974; 130:268–73.23. Crisel RM, Baker RS, Dorman DE. Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J Biol Chem. 1975; 250:4926–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection Rates of Bacteria in Chronic Otitis Media with Effusion in Children
- Middle ear histopathology in children with otitis media with effusion
- Detection of Haemophilus Influenzae and Streptococcus Pneumoniae by Polymerase Chain Reaction (PCR) in Chronic Otitis Media with Effusion (OME)
- Detection of Bacteria in the Middle Ear Effusion and Adenoid Tissue of Chronic Otitis Media Patient Using PCR Method
- Penetration of Cefprozil into Middle Ear Effusion in Pediatric Chronic Otitis Meida with Effusion