Arch Hand Microsurg.
2019 Dec;24(4):358-367. 10.12790/ahm.2019.24.4.358.
Robotic Microsurgery Training for Robot Assisted Reconstructive Surgery
- Affiliations
-
- 1Institute for Human Tissue Restoration, Department of Plastic and Reconstructive Surgery, Yonsei University College of Medicine, Seoul, Korea. hsaturn@hanmail.net
- KMID: 2464472
- DOI: http://doi.org/10.12790/ahm.2019.24.4.358
Abstract
- PURPOSE
Recent advances in robotic surgery have affected not only surgery for visceral organs but also head and neck cancer surgery and microsurgery. The authors intended to analyze and share experience gained from performing microanastomosis training in a new robotic surgery system.
METHODS
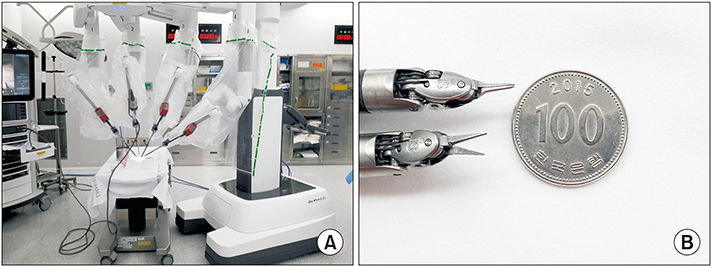
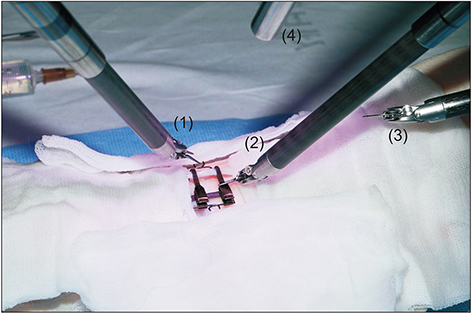
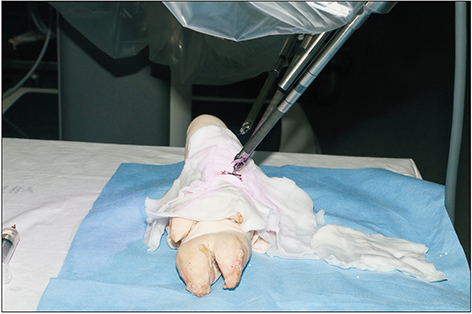
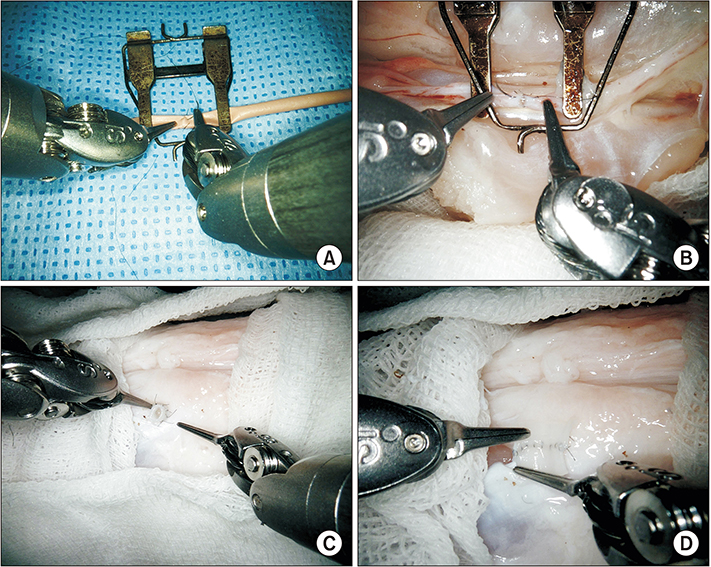
Robotic microanastomosis training was performed using Da Vinci Xi. The robot arm used two black diamond forceps, one Potts scissor, and one vision camera. First, basic robotic surgery skills were trained with Da Vinci Skill Simulator training. Actual microanastomosis practice was performed using artificial blood vessel, chicken wing and porcine leg.
RESULTS
Three simulation training sessions were performed and five vessel anastomosis were performed. A total of 8 vascular anastomosis were performed, and anastomosis for one vessel took 31-57 minutes. The number of sutures used was more than one initially due to suture material damage, but one suture was used after four anastomosis. In the anastomosis time analysis with porcine legs, the actual anastomosis process took 2 minutes 15 seconds±41 seconds per stitch. The vascular anastomosis interval took more time than vascular anastomosis itself due to robot arm change and camera movement.
CONCLUSION
Robotic microsurgery training was not difficult process for surgeons who had undergone conventional microsurgery. However, more training was needed to replace the robot arm and move the camera. In the long term, mechanical improvements in diamond forceps and camera resolution were necessary. In order to master robotic microsurgery, surgeons must get used to robotic surgery system through simulation training.
Keyword
MeSH Terms
Figure
Reference
-
1. Noaman HH. Microsurgery in children: history, indications, precautions, and differences from that of adults. Microsurgery. 2008; 28:83–84.
Article2. Krishnan KG, Dramm P, Schackert G. Simple and viable in vitro perfusion model for training microvascular anastomoses. Microsurgery. 2004; 24:335–338.
Article3. Garg A, Dwivedi RC, Sayed S, et al. Robotic surgery in head and neck cancer: a review. Oral Oncol. 2010; 46:571–576.
Article4. Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009; 31:283–289.
Article5. Kim WS, Jittreetat T, Nam W, Sannikorn P, Choi EC, Koh YW. Reconstruction of the segmental mandibular defect using a retroauricular or modified face-lift incision with an intraoral approach in head and neck cancer. Acta Otolaryngol. 2015; 135:500–506.
Article6. Hong JW, Kim YS, Lee WJ, Hong HJ, Roh TS, Song SY. Evaluation of the efficacy of microsurgical practice through time factor added protocol: microsurgical training using nonvital material. J Craniofac Surg. 2010; 21:876–881.7. Kwon SS, Jeong JH, Chang H, Minn KW. Training of microanastomosis with chicken wing brachial artery. J Korean Soc Plast Reconstr Surg. 2007; 34:274–277.8. Steffens K, Koob E, Hong G. Training in basic microsurgical techniques without experiments involving animals. Arch Orthop Trauma Surg. 1992; 111:198–203.
Article9. Weber D, Moser N, Rösslein R. A synthetic model for microsurgical training: a surgical contribution to reduce the number of animal experiments. Eur J Pediatr Surg. 1997; 7:204–206.
Article10. Saleh DB, Syed M, Kulendren D, Ramakrishnan V, Liverneaux PA. Plastic and reconstructive robotic microsurgery--a review of current practices. Ann Chir Plast Esthet. 2015; 60:305–312.
Article11. Karamanoukian RL, Bui T, McConnell MP, Evans GR, Karamanoukian HL. Transfer of training in robotic-assisted microvascular surgery. Ann Plast Surg. 2006; 57:662–665.
Article12. Selber JC, Alrasheed T. Robotic microsurgical training and evaluation. Semin Plast Surg. 2014; 28:5–10.13. Chalmers R, Schlabe J, Yeung E, Kerawala C, Cascarini L, Paleri V. Robot-assisted reconstruction in head and neck surgical oncology: the evolving role of the reconstructive microsurgeon. ORL J Otorhinolaryngol Relat Spec. 2018; 80:178–185.
Article14. van Mulken TJM, Boymans CAEM, Schols RM, et al. Preclinical experience using a new robotic system created for microsurgery. Plast Reconstr Surg. 2018; 142:1367–1376.
Article15. de Almeida JR, Genden EM. Robotic assisted reconstruction of the oropharynx. Curr Opin Otolaryngol Head Neck Surg. 2012; 20:237–245.
Article16. de Almeida JR, Park RC, Villanueva NL, Miles BA, Teng MS, Genden EM. Reconstructive algorithm and classification system for transoral oropharyngeal defects. Head Neck. 2014; 36:934–941.
Article17. Loevner LA, Learned KO, Mohan S, et al. Transoral robotic surgery in head and neck cancer: what radiologists need to know about the cutting edge. Radiographics. 2013; 33:1759–1779.
Article18. Song HG, Yun IS, Lee WJ, Lew DH, Rah DK. Robotassisted free flap in head and neck reconstruction. Arch Plast Surg. 2013; 40:353–358.
Article19. Arora R, Verma VK, Mishra KS, Bhoye H, Kapoor R. Reconstruction with free flaps in robotic head-and-neck onco-surgeries. Indian J Plast Surg. 2018; 51:283–289.
Article20. Hassanein AH, Mailey BA, Dobke MK. Robot-assisted plastic surgery. Clin Plast Surg. 2012; 39:419–424.
Article21. Nehme J, Neville JJ, Bahsoun AN. The use of robotics in plastic and reconstructive surgery: a systematic review. JPRAS Open. 2017; 13:1–10.