Cancer Res Treat.
2019 Apr;51(2):685-695. 10.4143/crt.2018.250.
Reduction of Target Volume and the Corresponding Dose for the Tumor Regression Field after Induction Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, National Cancer Center/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China.
- 2Department of Radiation Oncology, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.
- 3Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China. suyong@sysucc.org.cn
- 4Department of Radiotherapy, TCM-Integrated Cancer Center of Southern Medical University, Guangzhou, China.
- 5Department of Oncology, Shunde Hospital, Southern Medical University, Foshan, China.
- KMID: 2464414
- DOI: http://doi.org/10.4143/crt.2018.250
Abstract
- PURPOSE
This study aims to investigate the feasibility of contouring target volume according to residual tumor and decreasing the dose to the tumor regression field after induction chemotherapy (IC) in locoregionally advanced nasopharyngeal carcinoma (NPC).
MATERIALS AND METHODS
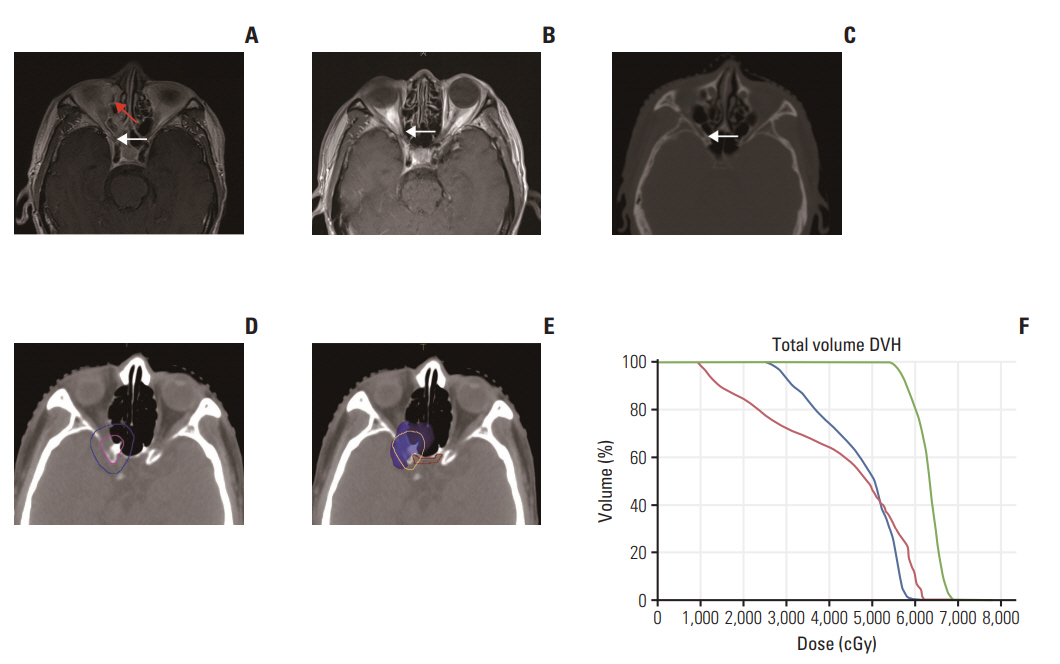
From August 2009 to August 2013, patients with stage III-IVB NPC were treated with IC and concurrent chemoradiotherapy. Gross tumor volume of nasopharynx (GTVnx)-residual and gross tumor volume of cervical lymph node (GTVnd)-residual were contoured according to post-IC residual primary tumor and any N+ disease, respectively. The tumor regression field was included in CTVnx1/CTVnd1 and prescribed a dose of 60 Gy. Outcomes and toxicities of all patients were evaluated.
RESULTS
A total of 57 patients were enrolled. At a median follow-up of 68 months, three cases displayed locoregional recurrence and one case showed both distant metastasis and locoregional recurrence. All locoregional recurrences were in the GTVnx-residual/GTVnd-residual and in-field. The 5-year overall, locoregional relapse-free, distant metastasis-free, and progression-free survival rates were 82.2%, 87.7%, 85.8% and 80.3%, respectively.
CONCLUSION
After IC, contouring of GTVnx-residual/GTVnd-residual as residual tumor volume and distribution 60 Gy ofradiation dose to the tumorregression field may be feasible and need further investigation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Razak AR, Siu LL, Liu FF, Ito E, O'Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010; 46:1967–78.
Article2. Pfister DG, Spencer S, Adelstein D, Adkins D, Brizel DM, Burtness BB, et al. NCCN clinical practice guidelines in oncology (NCCN Guidelines): head and neck cancers. Version 2.2016-October 11, 2016. Fort Washington, PA: National Comprehensive Cancer Network;2016.3. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016; 17:1509–20.4. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009; 27:242–9.
Article5. Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 2012; 23:427–35.
Article6. Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui NJ, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001; 19:1350–7.
Article7. Halperin EC, Brady LW, Perez CA, Wazer DE. Perez and Brady’s principles and practice of radiation oncology. 6th ed. Philadelphia, PA: Lippincott Williams &Wilkins;2013.8. Salama JK, Haddad RI, Kies MS, Busse PM, Dong L, Brizel DM, et al. Clinical practice guidance for radiotherapy planning after induction chemotherapy in locoregionally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009; 75:725–33.
Article9. Illidge T, Specht L, Yahalom J, Aleman B, Berthelsen AK, Constine L, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2014; 89:49–58.
Article10. Kalemkerian GP, Loo BW Jr, Akerley W, Attia A, Chow LQ, Decker R. NCCN clinical practice guidelines in oncology (NCCN guidelines): small cell lung cancer. Version 3.2017-February 23, 2017. Fort Washington, PA: National Comprehensive Cancer Network;2017.11. De Ruysscher D, Faivre-Finn C, Nestle U, Hurkmans CW, Le Pechoux C, Price A, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol. 2010; 28:5301–10.
Article12. Sun Y, Yu XL, Luo W, Lee AW, Wee JT, Lee N, et al. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensitymodulated radiotherapy. Radiother Oncol. 2014; 110:390–7.
Article13. Sun Y, Yu XL, Zhang GS, Liu YM, Tao CJ, Guo R, et al. Reduction of clinical target volume in patients with lateralized cancer of the nasopharynx and without contralateral lymph node metastasis receiving intensity-modulated radiotherapy. Head Neck. 2016; 38 Suppl 1:E468–72.
Article14. Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011; 117:1874–83.15. RTOG Foundation. RTOG 0225 protocol information [Internet]. Rockville, MD: National Cancer Institute;2008. [cited 2018 Jul 2]. Available from: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0225.16. Kong F, Ying H, Du C, Huang S, Zhou J, Chen J, et al. Patterns of local-regional failure after primary intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiat Oncol. 2014; 9:60.
Article17. Fletcher GH. Clinical dose response curves of human malignant epithelial tumours. Br J Radiol. 1973; 46:151.18. Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an openlabel, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018; 19:461–73.
Article19. Girinsky T, van der Maazen R, Specht L, Aleman B, Poortmans P, Lievens Y, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol. 2006; 79:270–7.
Article20. Verhappen MH, Poortmans PM, Raaijmakers E, Raemaekers JM. Reduction of the treated volume to involved node radiation therapy as part of combined modality treatment for early stage aggressive non-Hodgkin's lymphoma. Radiother Oncol. 2013; 109:133–9.
Article21. Girinsky T, Specht L, Ghalibafian M, Edeline V, Bonniaud G, Van Der Maazen R, et al. The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol. 2008; 88:202–10.
Article22. Maraldo MV, Aznar MC, Vogelius IR, Petersen PM, Specht L. Involved node radiation therapy: an effective alternative in early-stage hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2013; 85:1057–65.
Article23. De Ruysscher D, Faivre-Finn C, Moeller D, Nestle U, Hurkmans CW, Le Pechoux C, et al. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother Oncol. 2017; 124:1–10.
Article24. Yu Z, Luo W, Zhou QC, Zhang QH, Kang DH, Liu MZ. Impact of changing gross tumor volume delineation of intensity-modulated radiotherapy on the dose distribution and clinical treatment outcome after induction chemotherapy for the primary locoregionally advanced nasopharyngeal carcinoma. Ai Zheng. 2009; 28:1132–7.
Article25. Xue F, Hu C, He X. Induction chemotherapy followed by intensity-modulated radiotherapy with reduced gross tumor volume delineation for stage T3-4 nasopharyngeal carcinoma. Onco Targets Ther. 2017; 10:3329–36.
Article26. Yang H, Chen X, Lin S, Rong J, Yang M, Wen Q, et al. Treatment outcomes after reduction of the target volume of intensity-modulated radiotherapy following induction chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a prospective, multi-center, randomized clinical trial. Radiother Oncol. 2018; 126:37–42.
Article27. Zhou GQ, Yu XL, Chen M, Guo R, Lei Y, Sun Y, et al. Radiation-induced temporal lobe injury for nasopharyngeal carcinoma: a comparison of intensity-modulated radiotherapy and conventional two-dimensional radiotherapy. PLoS One. 2013; 8:e67488.
Article28. Su SF, Huang SM, Han F, Huang Y, Chen CY, Xiao WW, et al. Analysis of dosimetric factors associated with temporal lobe necrosis (TLN) in patients with nasopharyngeal carcinoma (NPC) after intensity modulated radiotherapy. Radiat Oncol. 2013; 8:17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Less is more: role of additional chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal cancer management
- Locoregionally advanced nasopharyngeal carcinoma treated with intensity-modulated radiotherapy plus concurrent weekly cisplatin with or without neoadjuvant chemotherapy
- Induction Chemotherapy Plus Concurrent Chemoradiotherapy Versus Concurrent Chemoradiotherapy Alone in Locoregionally Advanced Nasopharyngeal Carcinoma in Children and Adolescents: A Matched Cohort Analysis
- Organ Preservation for the Management of Locally Advanced Head and Neck Cancer
- Prognostic Value of Serum Epstein-Barr Virus Antibodies and Their Correlation with TNM Classification in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma