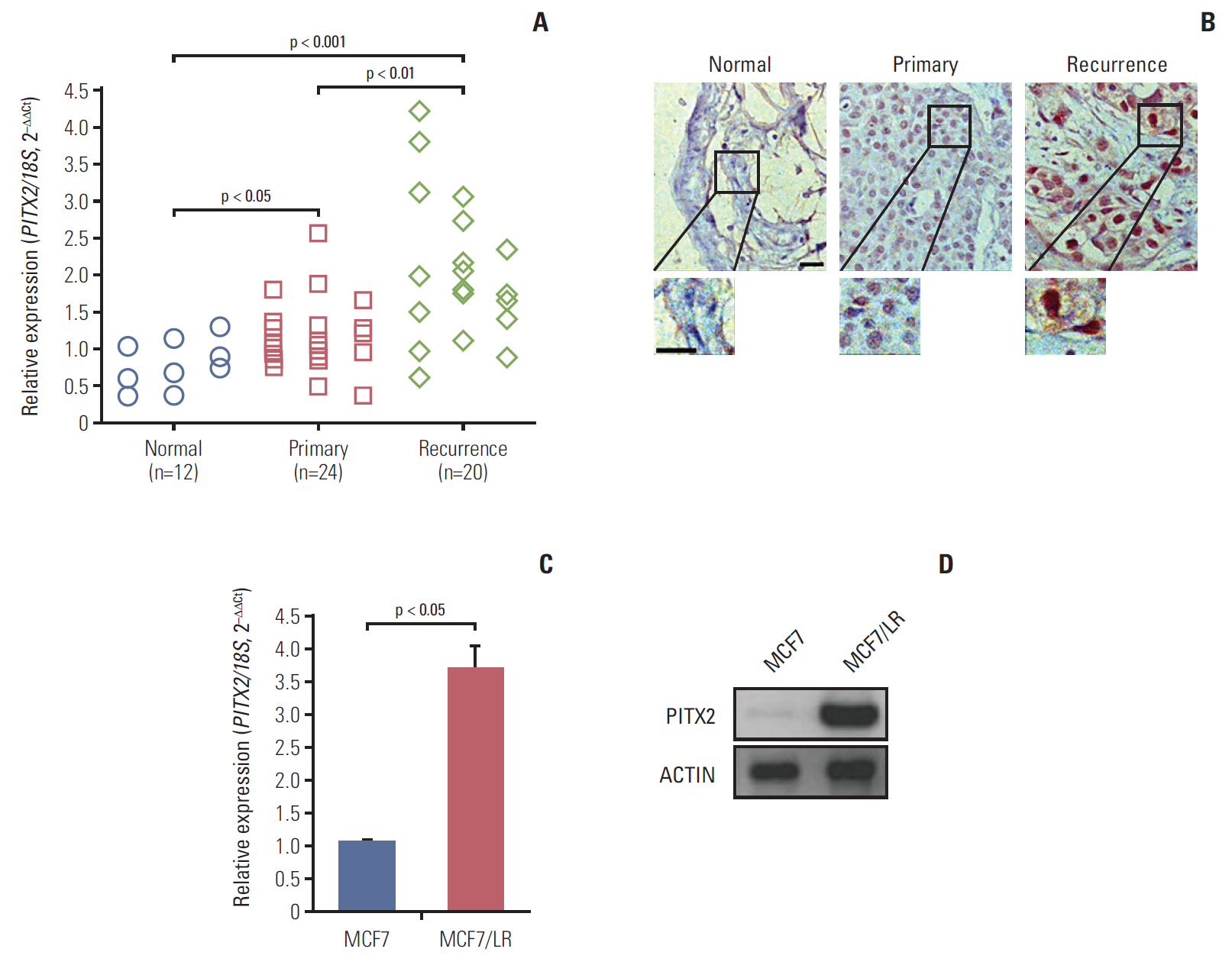
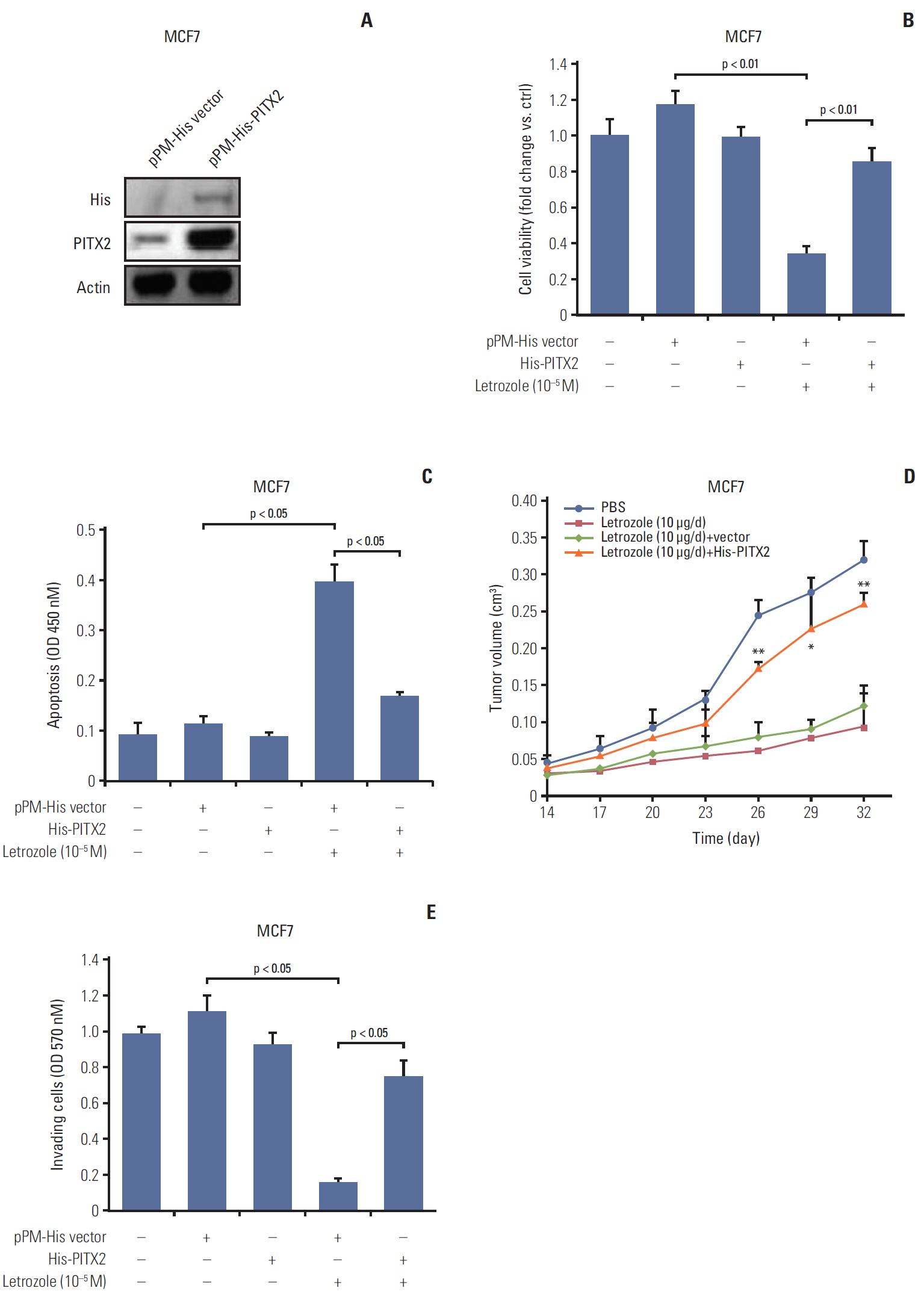
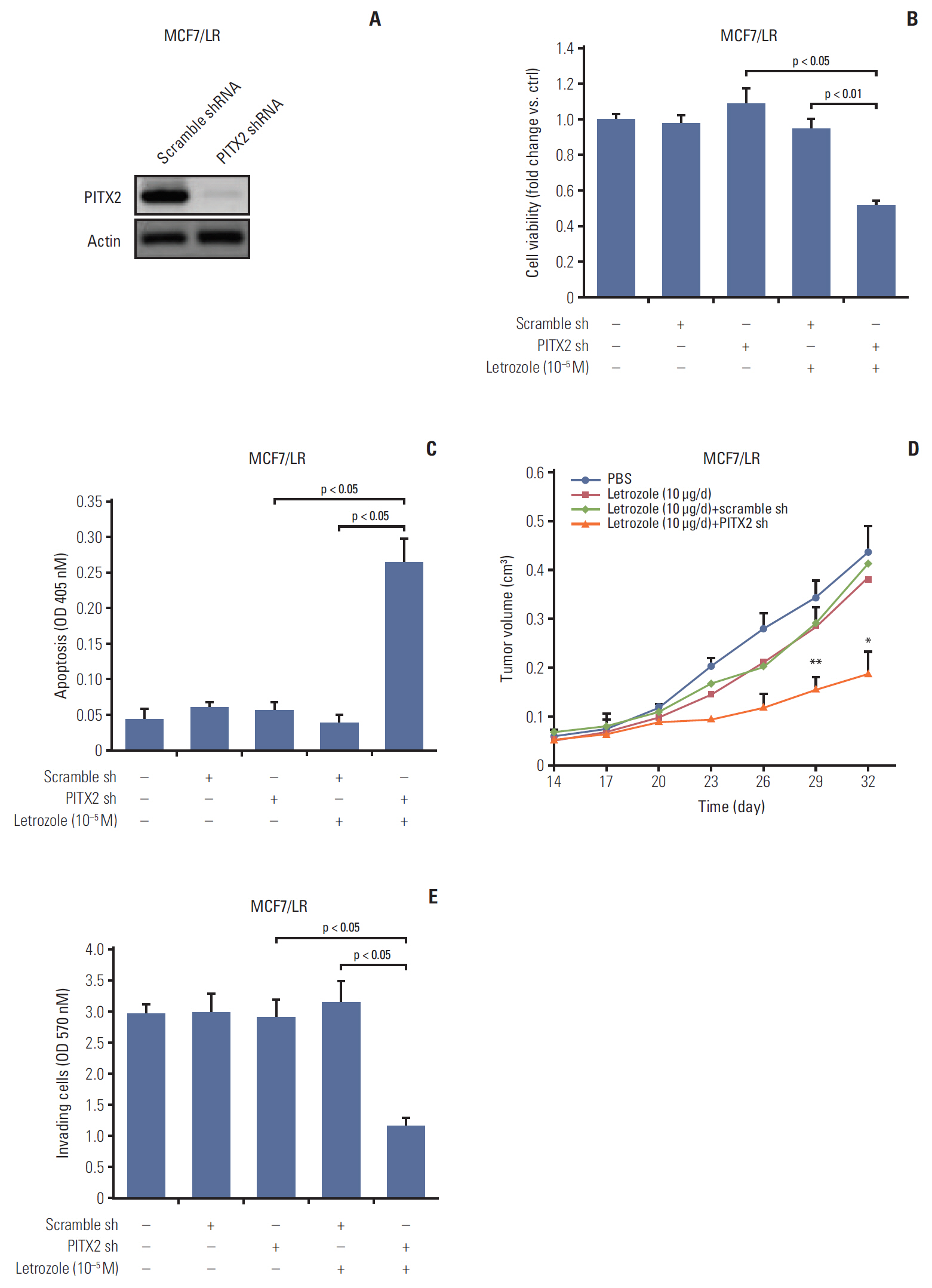
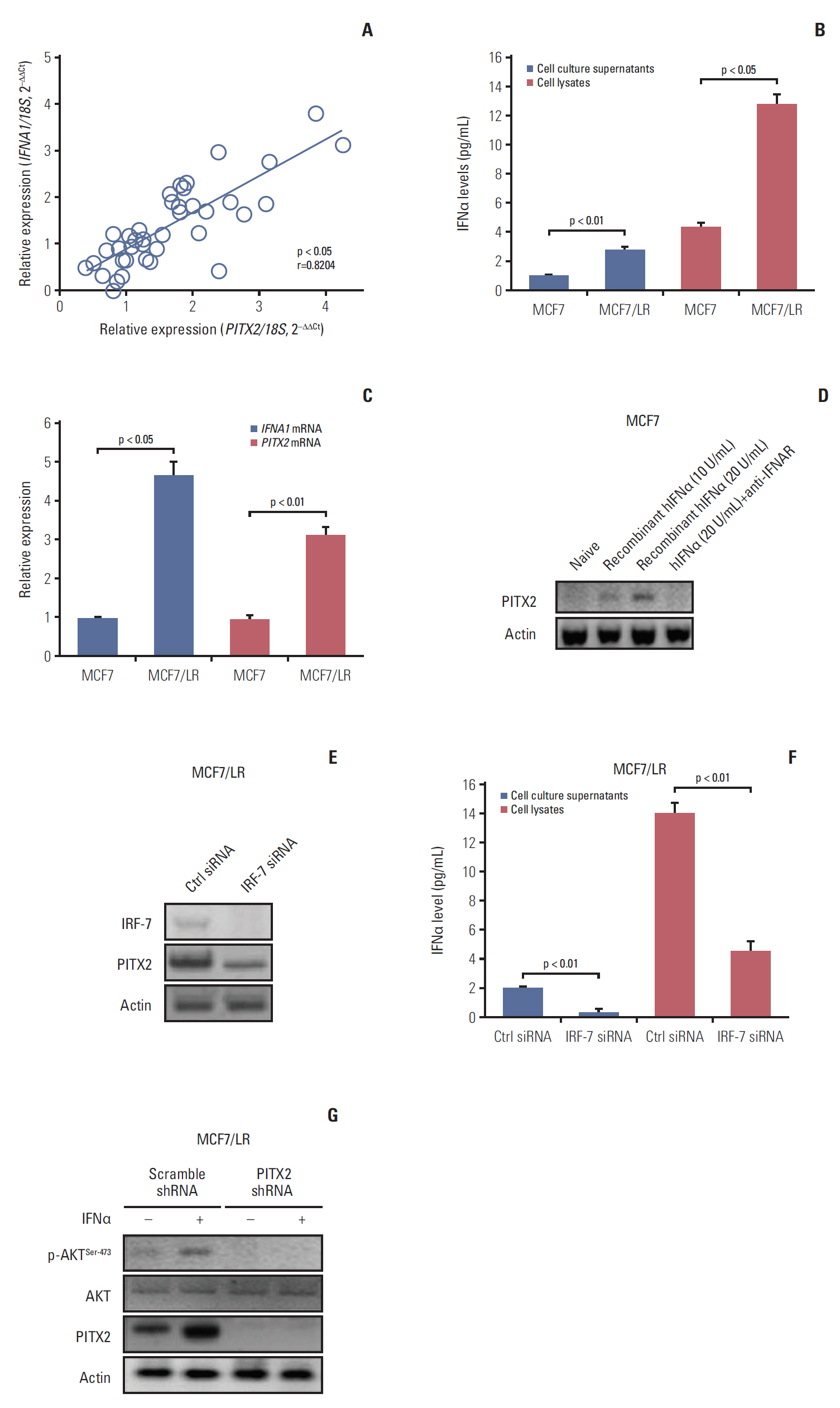
Cancer Res Treat.
2019 Apr;51(2):576-592. 10.4143/crt.2018.100.
Upregulation of PITX2 Promotes Letrozole Resistance Via Transcriptional Activation of IFITM1 Signaling in Breast Cancer Cells
- Affiliations
-
- 1Department of Breast Surgery, First Affiliated Hospital of China Medical University, Shenyang, China.
- 2Department of Medical Imaging, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, China.
- 3Department of Breast Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, China. zhangqiang8220@163.com
- KMID: 2464405
- DOI: http://doi.org/10.4143/crt.2018.100
Abstract
- PURPOSE
Although the interferon α (IFNα) signaling and the paired-like homeodomain transcription factor 2 (PITX2) have both been implicated in the progression of breast cancer (BCa), it remains obscure whether these two pathways act in a coordinated manner. We therefore aimed to elucidate the expression and function of PITX2 during the pathogenesis of endocrine resistance in BCa.
MATERIALS AND METHODS
PITX2 expression was assessed in BCa tissues using quantitative reverse transcription polymerase chain reaction (RT-qPCR) and immunohistochemistry and in experimentally induced letrozole-resistant BCa cells using RT-qPCR and immunoblotting. Effects of PITX2 deregulation on BCa progression was determined by assessing MTT, apoptosis and xenograft model. Finally, using multiple assays, the transcriptional regulation of interferon-inducible transmembrane protein 1 (IFITM1) by PITX2 was studied at both molecular and functional levels.
RESULTS
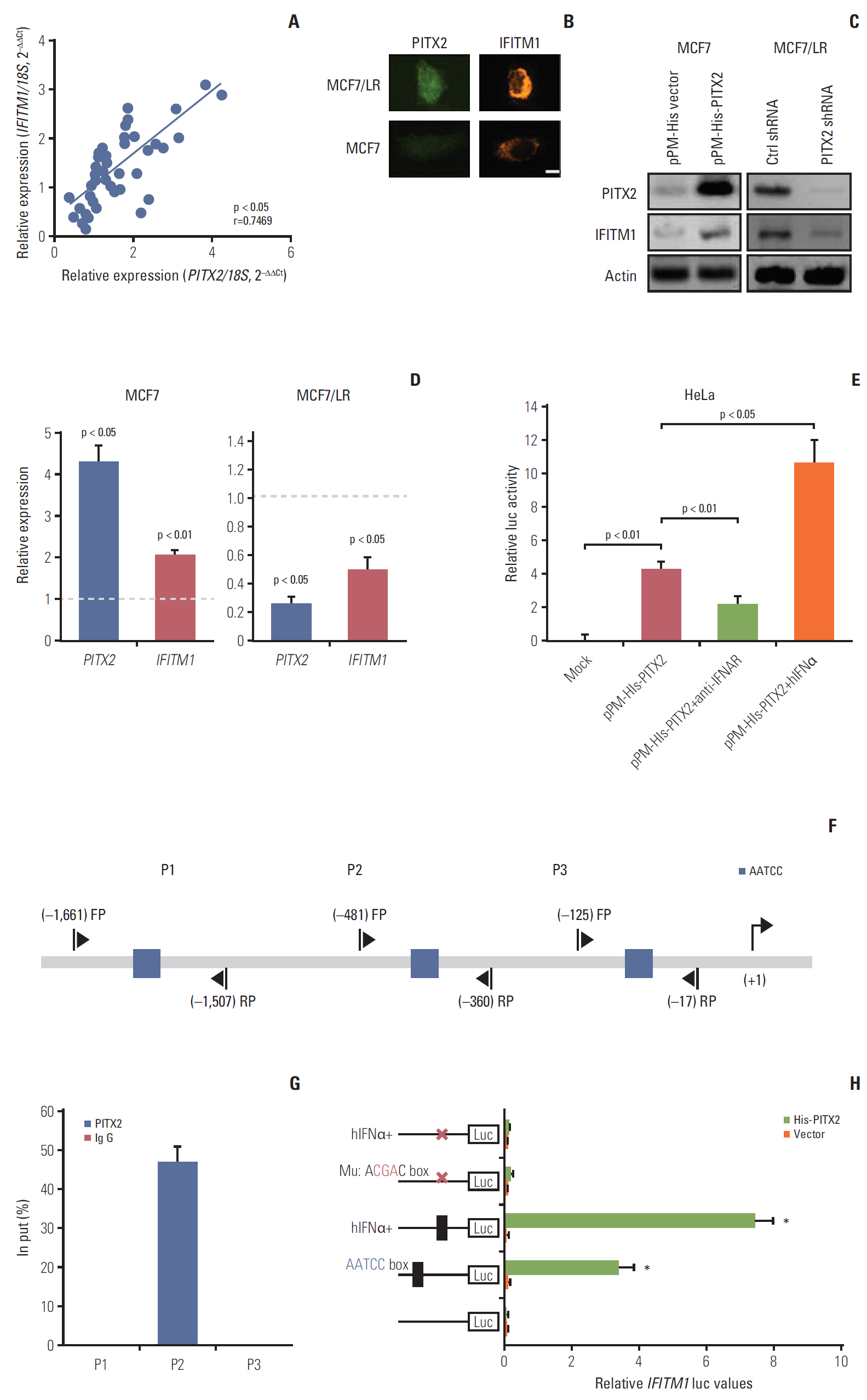
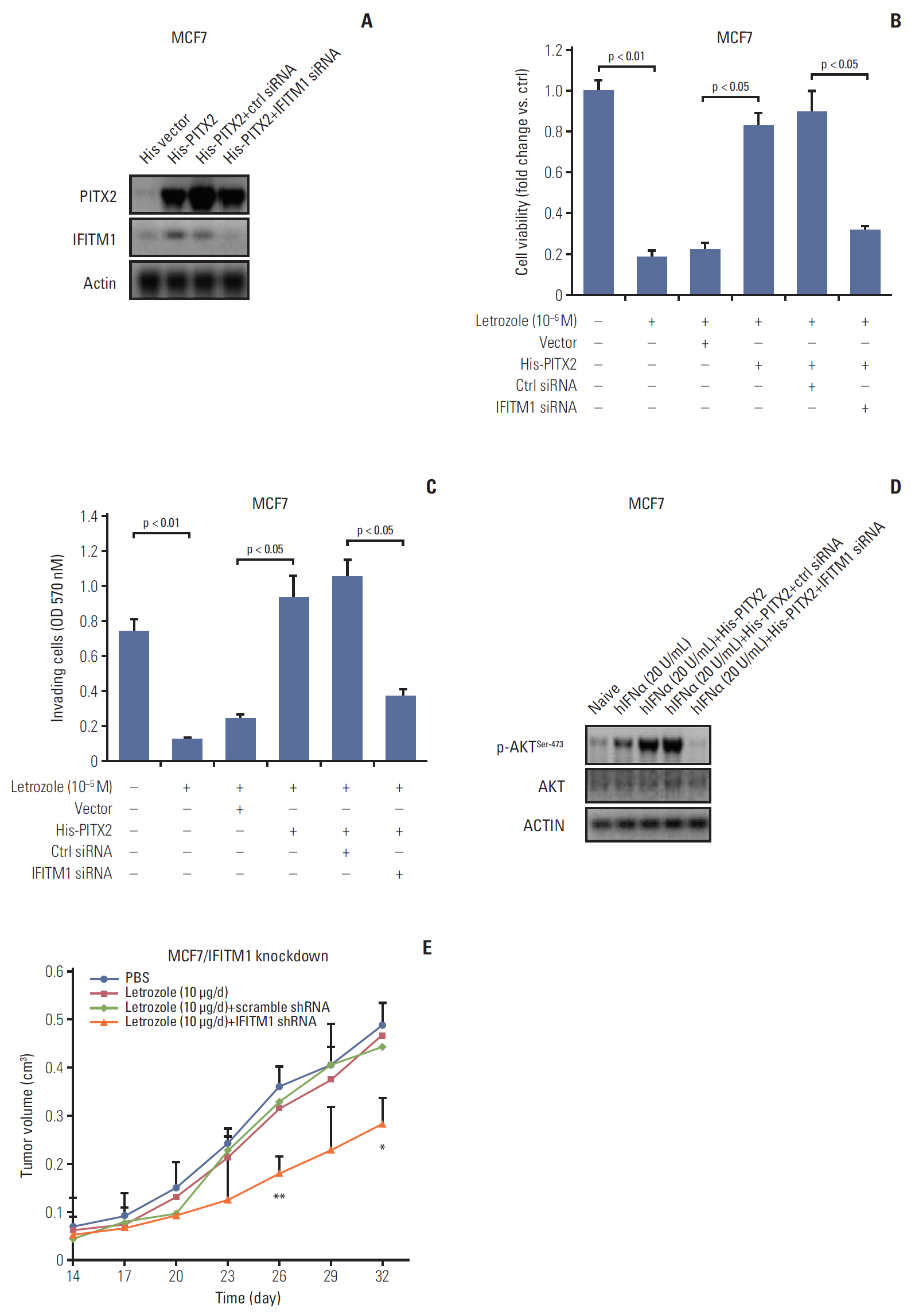
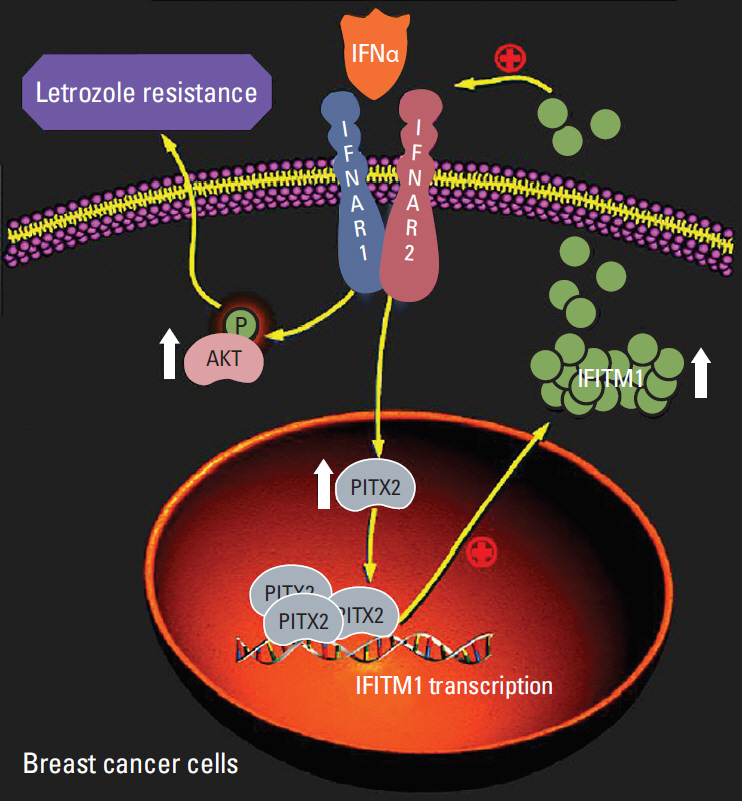
PITX2 expression was induced in letrozole-resistant BCa tissues and cells, and PITX2 induction by IFNα signaling powerfully protected BCa cells against letrozole insult and potentiated letrozole-resistance. Mechanistically, PITX2 enhanced IFNα-induced AKT activation by transactivating the transcription of IFITM1, thus rendering BCa cells unresponsive to letrozoleelicited cell death. Additionally, ablation of IFITM1 expression using siRNA substantially abolished IFNα-elicited AKT phosphorylation, even in the presence of PITX2 overexpression, thus sensitizing BCa cells to letrozole treatment.
CONCLUSION
These results demonstrate that constitutive upregulation of PITX2/IFITM1 cascade is an intrinsic adaptive mechanism during the pathogenesis of letrozole-resistance, and modulation of PITX2/IFITM1 level using different genetic and pharmacological means would thus have a novel therapeutic potential against letrozole resistance in BCa.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Hoeflich KP, Guan J, Edgar KA, O'Brien C, Savage H, Wilson TR, et al. The PI3K inhibitor taselisib overcomes letrozole resistance in a breast cancer model expressing aromatase. Genes Cancer. 2016; 7:73–85.
Article2. Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res. 2005; 65:5380–9.
Article3. Brodie AM, Chumsri S, Sukumar S, Sabnis GJ. Extending aromatase inhibitor sensitivity in hormone resistant breast cancer. Horm Mol Biol Clin Investig. 2011; 5:97–103.
Article4. Choi HJ, Lui A, Ogony J, Jan R, Sims PJ, Lewis-Wambi J. Targeting interferon response genes sensitizes aromatase inhibitor resistant breast cancer cells to estrogen-induced cell death. Breast Cancer Res. 2015; 17:6.
Article5. Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci U S A. 2011; 108:18879–86.
Article6. Zacharias AL, Lewandoski M, Rudnicki MA, Gage PJ. Pitx2 is an upstream activator of extraocular myogenesis and survival. Dev Biol. 2011; 349:395–405.
Article7. Dietrich D, Hasinger O, Liebenberg V, Field JK, Kristiansen G, Soltermann A. DNA methylation of the homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell lung cancer patients. Diagn Mol Pathol. 2012; 21:93–104.
Article8. Lian ZQ, Wang Q, Li WP, Zhang AQ, Wu L. Screening of significantly hypermethylated genes in breast cancer using microarray-based methylated-CpG island recovery assay and identification of their expression levels. Int J Oncol. 2012; 41:629–38.
Article9. Vasiljevic N, Ahmad AS, Carter PD, Fisher G, Berney DM, Foster CS, et al. DNA methylation of PITX2 predicts poor survival in men with prostate cancer. Biomark Med. 2014; 8:1143–50.10. Zhang JX, Tong ZT, Yang L, Wang F, Chai HP, Zhang F, et al. PITX2: a promising predictive biomarker of patients' prognosis and chemoradioresistance in esophageal squamous cell carcinoma. Int J Cancer. 2013; 132:2567–77.
Article11. Lee WK, Chakraborty PK, Thevenod F. Pituitary homeobox 2 (PITX2) protects renal cancer cell lines against doxorubicin toxicity by transcriptional activation of the multidrug transporter ABCB1. Int J Cancer. 2013; 133:556–67.
Article12. Yang Z, Li C, Fan Z, Liu H, Zhang X, Cai Z, et al. Single-cell sequencing reveals variants in ARID1A, GPRC5A and MLL2 driving self-renewal of human bladder cancer stem cells. Eur Urol. 2017; 71:8–12.
Article13. Gu F, Hsu HK, Hsu PY, Wu J, Ma Y, Parvin J, et al. Inference of hierarchical regulatory network of estrogen-dependent breast cancer through ChIP-based data. BMC Syst Biol. 2010; 4:170.
Article14. Yu T, Yu HR, Sun JY, Zhao Z, Li S, Zhang XF, et al. miR-1271 inhibits ERalpha expression and confers letrozole resistance in breast cancer. Oncotarget. 2017; 8:107134–48.15. Dong YS, Hou WG, Li Y, Liu DB, Hao GZ, Zhang HF, et al. Unexpected requirement for a binding partner of the syntaxin family in phagocytosis by murine testicular Sertoli cells. Cell Death Differ. 2016; 23:787–800.
Article16. Zhang C, Lai JH, Hu B, Zhang S, Zhao J, Li W. A chromatin modifier regulates Sertoli cell response to mono-(2-ethylhexyl) phthalate (MEHP) via tissue inhibitor of metalloproteinase 2 (TIMP2) signaling. Biochim Biophys Acta. 2014; 1839:1170–82.
Article17. Zhang Q, Liu XY, Li S, Zhao Z, Li J, Cui MK, et al. Repression of ESR1 transcription by MYOD potentiates letrozole-resistance in ERalpha-positive breast cancer cells. Biochem Biophys Res Commun. 2017; 492:425–33.18. Ning P, Zhong JG, Jiang F, Zhang Y, Zhao J, Tian F, et al. Role of protein S in castration-resistant prostate cancer-like cells. Endocr Relat Cancer. 2016; 23:595–607.
Article19. Lopez JI, Angulo JC, Martin A, Sanchez-Chapado M, Gonzalez-Corpas A, Colas B, et al. A DNA hypermethylation profile reveals new potential biomarkers for the evaluation of prognosis in urothelial bladder cancer. APMIS. 2017; 125:787–96.
Article20. Kapoor S. Promising, new prognostic markers of esophageal carcinomas. APMIS. 2013; 121:1011.
Article21. Liu Y, Nan F, Lu K, Wang Y, Liu Y, Wei S, et al. Identification of key genes in endometrioid endometrial adenocarcinoma via TCGA database. Cancer Biomark. 2017; 21:11–21.
Article22. Vela I, Morrissey C, Zhang X, Chen S, Corey E, Strutton GM, et al. PITX2 and non-canonical Wnt pathway interaction in metastatic prostate cancer. Clin Exp Metastasis. 2014; 31:199–211.
Article23. Wan Abdul Rahman WF, Fauzi MH, Jaafar H. Expression of DNA methylation marker of paired-like homeodomain transcription factor 2 and growth receptors in invasive ductal carcinoma of the breast. Asian Pac J Cancer Prev. 2014; 15:8441–5.24. Jezkova E, Kajo K, Zubor P, Grendar M, Malicherova B, Mendelova A, et al. Methylation in promoter regions of PITX2 and RASSF1A genes in association with clinicopathological features in breast cancer patients. Tumour Biol. 2014; 37:15707–18.
Article25. Gultekin M, Eren G, Babacan T, Yildiz F, Altundag K, Guler N, et al. Metaplastic breast carcinoma: a heterogeneous disease. Asian Pac J Cancer Prev. 2014; 15:2851–6.
Article26. Khan MS, Pandith AA, Masoodi SR, Wani KA, Ul Hussain M, Mudassar S. Epigenetic silencing of TSHR gene in thyroid cancer patients in relation to their BRAF V600E mutation status. Endocrine. 2014; 47:449–55.
Article27. Zhang JX, Chen ZH, Xu Y, Chen JW, Weng HW, Yun M, et al. Downregulation of microRNA-644a promotes esophageal squamous cell carcinoma aggressiveness and stem cell-like phenotype via dysregulation of PITX2. Clin Cancer Res. 2017; 23:298–310.
Article28. Torrado M, Franco D, Lozano-Velasco E, Hernandez-Torres F, Calvino R, Aldama G, et al. A microRNA-transcription factor blueprint for early atrial arrhythmogenic remodeling. Biomed Res Int. 2015; 2015:263151.
Article29. Cavazzoni A, Bonelli MA, Fumarola C, La Monica S, Airoud K, Bertoni R, et al. Overcoming acquired resistance to letrozole by targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones. Cancer Lett. 2012; 323:77–87.
Article30. Lei H, Furlong PJ, Ra JH, Mullins D, Cantor R, Fraker DL, et al. AKT activation and response to interferon-beta in human cancer cells. Cancer Biol Ther. 2005; 4:709–15.31. Li P, Shi ML, Shen WL, Zhang Z, Xie DJ, Zhang XY, et al. Coordinated regulation of IFITM1, 2 and 3 genes by an IFN-responsive enhancer through long-range chromatin interactions. Biochim Biophys Acta Gene Regul Mech. 2017; 1860:885–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Effect of Letrozole on Gastric Cancer?
- High Levels of Hyaluronic Acid Synthase-2 Mediate NRF2-Driven Chemoresistance in Breast Cancer Cells
- p90RSK Activation Promotes Epithelial-Mesenchymal Transition in Cisplatin-Treated Triple-Negative Breast Cancer Cells
- New Findings on Breast Cancer Stem Cells: A Review
- Nkx-2.5 Regulates MDR1 Expression via Its Upstream Promoter in Breast Cancer Cells