Cancer Res Treat.
2019 Apr;51(2):464-473. 10.4143/crt.2018.186.
Combination Treatment of Stereotactic Body Radiation Therapy and Immature Dendritic Cell Vaccination for Augmentation of Local and Systemic Effects
- Affiliations
-
- 1Department of Radiation Oncology, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea. drasdf19@gmail.com
- 2Department of Microbiology, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Research Center, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea.
- KMID: 2464393
- DOI: http://doi.org/10.4143/crt.2018.186
Abstract
- PURPOSE
The purpose of this study was to investigate the efficacy of stereotactic body radiation therapy (SBRT) as a tumor-associated antigen (TAA) presentation method for dendritic cell (DC) sensitization and evaluate its effect in combination with immunotherapy using an intratumoral injection of immature DCs (iDCs).
MATERIALS AND METHODS
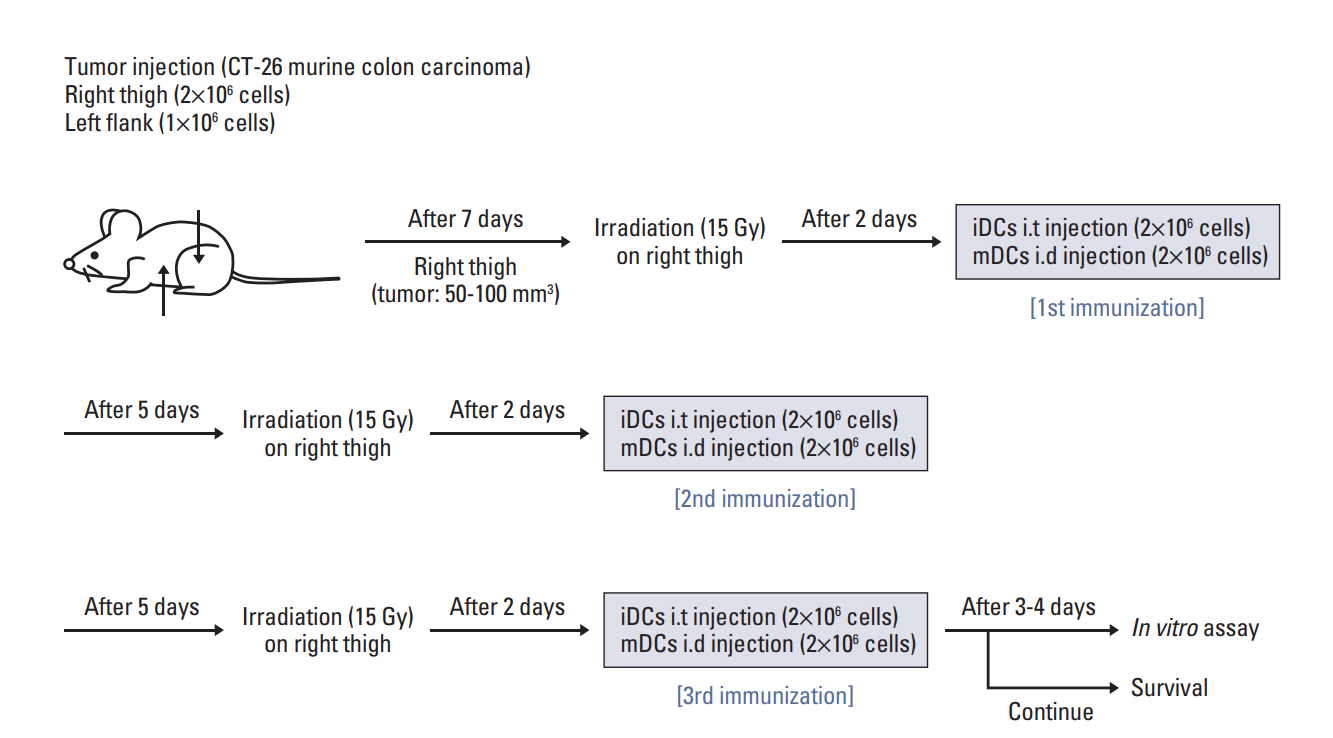
CT-26 colon carcinoma cell was used as a cancer cell line. Annexin V staining and phagocytosis assays were performed to determine the appropriate radiation dose and incubation time to generate TAAs. BALB/c mice were used for in vivo experiments. Cancer cells were injected into the right legs and left flanks to generate primary and metastatic tumors, respectively. The mice were subjected to radiation therapy (RT) alone, intradermal injection of electroporated DCs alone, or RT in combination with iDC intratumoral injection (RT/iDC). Tumor growth measurement and survival rate analysis were performed. Enzyme-linked immunospot and cytotoxicity assays were performed to observe the effect of different treatments on the immune system.
RESULTS
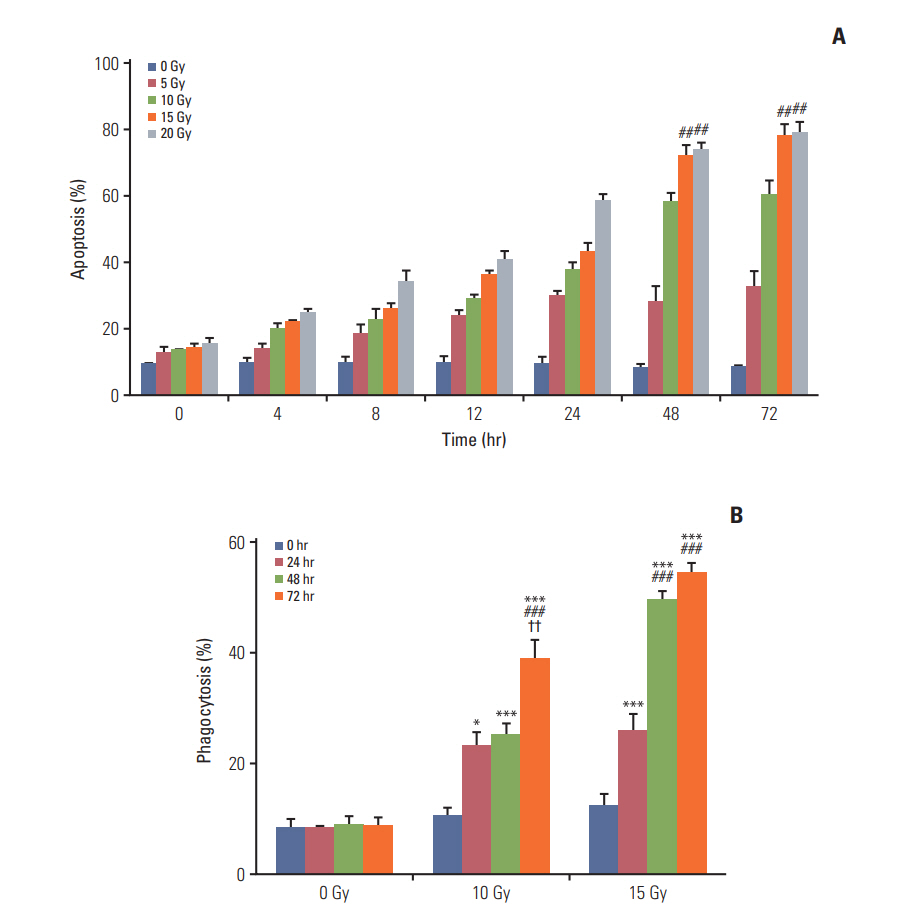
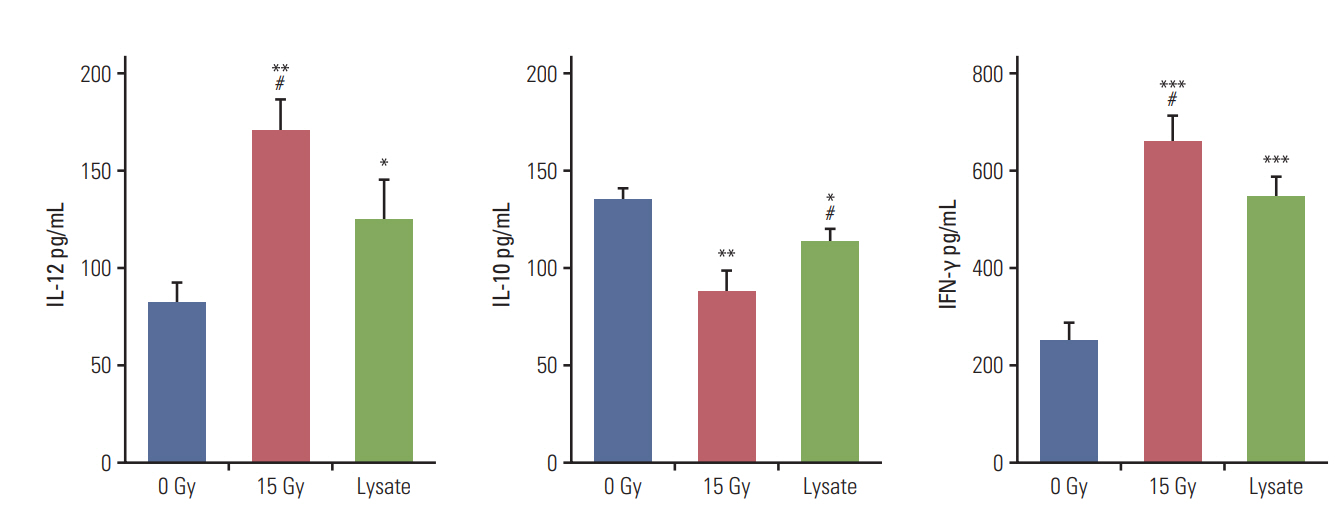
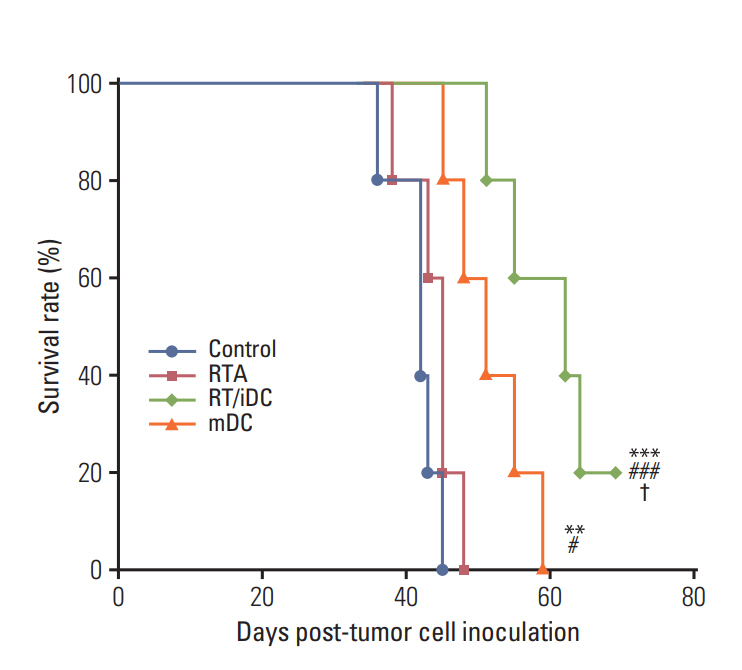
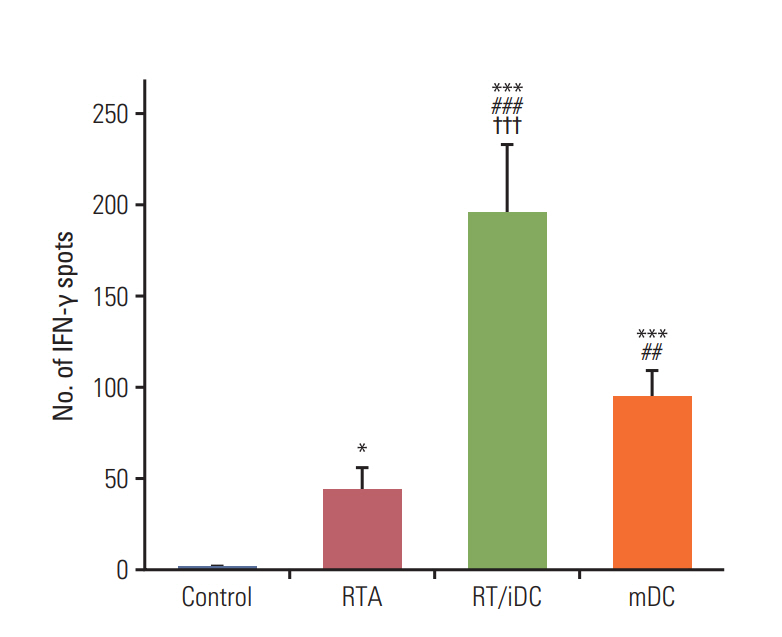
Annexin V staining and phagocytosis assays showed that 15 Gy radiation dose and 48 hours of incubation was appropriate for subsequent experiments. Maximum DC sensitization and T-cell stimulation was observed with RT as compared to other TAA preparation methods. In vivo assays revealed statistically significant delay in the growth of both primary and metastatic tumors in the RT/iDC group. The overall survival rate was the highest in the RT/iDC group.
CONCLUSION
The combination of SBRT and iDC vaccination may enhance treatment effects. Clinical trials and further studies are warranted in the future.
MeSH Terms
Figure
Reference
-
References
1. Datta J, Terhune JH, Lowenfeld L, Cintolo JA, Xu S, Roses RE, et al. Optimizing dendritic cell-based approaches for cancer immunotherapy. Yale J Biol Med. 2014; 87:491–518.2. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012; 12:265–77.
Article3. Zarnani AH, Torabi-Rahvar M, Bozorgmehr M, Zareie M, Mojtabavi N. Improved efficacy of a dendritic cell-based vaccine against a murine model of colon cancer: the helper protein effect. Cancer Res Treat. 2015; 47:518–26.
Article4. Kim SK, Yun CH, Han SH. Enhanced anti-cancer activity of human dendritic cells sensitized with gamma-irradiationinduced apoptotic colon cancer cells. Cancer Lett. 2013; 335:278–88.
Article5. Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A. 2002; 99:931–6.
Article6. Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998; 95:9482–7.7. Son CH, Bae JH, Shin DY, Lee HR, Yang K, Park YS. Antitumor effect of dendritic cell loaded ex vivo and in vivo with tumor-associated antigens in lung cancer model. Immunol Invest. 2014; 43:447–62.8. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004; 58:862–70.
Article9. Koo T, Kim IA. Radiotherapy and immune checkpoint blockades: a snapshot in 2016. Radiat Oncol J. 2016; 34:250–9.
Article10. Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015; 356:82–90.
Article11. Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007; 83:819–25.
Article12. Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern radiotherapy concepts and the impact of radiation on immune activation. Front Oncol. 2016; 6:141.
Article13. Derer A, Deloch L, Rubner Y, Fietkau R, Frey B, Gaipl US. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses: pre-clinical evidence and ongoing clinical applications. Front Immunol. 2015; 6:505.
Article14. O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998; 8:275–83.15. Igietseme JU, Ananaba GA, Bolier J, Bowers S, Moore T, Belay T, et al. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J Immunol. 2000; 164:4212–9.
Article16. Weiss EM, Frey B, Rodel F, Herrmann M, Schlucker E, Voll RE, et al. Ex vivo- and in vivo-induced dead tumor cells as modulators of antitumor responses. Ann N Y Acad Sci. 2010; 1209:109–17.17. Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005; 28:129–35.
Article18. Slota M, Lim JB, Dang Y, Disis ML. ELISpot for measuring human immune responses to vaccines. Expert Rev Vaccines. 2011; 10:299–306.
Article19. Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010; 37:4078–101.
Article20. Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016; 4:51.
Article21. Sharabi AB, Tran PT, Lim M, Drake CG, Deweese TL. Stereotactic radiation therapy combined with immunotherapy: augmenting the role of radiation in local and systemic treatment. Oncology (Williston Park). 2015; 29:331–40.22. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013; 31:140–4.
Article23. Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013; 4:e631.
Article24. Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003; 283:1–16.
Article25. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010; 11:700–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Top Ten Lessons Learned from Trials in Oligometastatic Cancers
- Role of Radiation Therapy for Non-small Cell Lung Cancer: Focused on Stereotactic Ablative Radiation Therapy in Stage I
- Stereotactic Body Radiotherapy for Early Stage Lung Cancer
- A Case of Achieving Complete Remission with Stereotactic Body Radiation Therapy in Patients with Hepatocellular Carcinoma with Macrovascular Invasion after Repeated Transarerial Chemoembolization
- Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma