Nutr Res Pract.
2019 Oct;13(5):377-383. 10.4162/nrp.2019.13.5.377.
Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice
- Affiliations
-
- 1Department of Food and Nutrition, Kyung Hee University, 26 Kyunghee-Daero, Dongdaemun-Gu, Seoul 02447, Republic of Korea. ylim@khu.ac.kr
- KMID: 2464116
- DOI: http://doi.org/10.4162/nrp.2019.13.5.377
Abstract
- BACKGROUND/OBJECTIVES
Hyperglycemia-induced hepatic damage has been recognized as one of the major cause of complications in diabetes. Hepatic complications are associated with inflammation and oxidative stress in diabetes. In this study, we investigated the hypothesis that gamma-tocopherol (GT) supplementation ameliorates NLRP3 inflammasome associated hepatic inflammation in diabetes.
MATERIALS/METHODS
Diabetes was induced by the intraperitoneal injection of alloxan (150 mg/kg. BW) in ICR mice. All mice were fed with a control diet (AIN-76A). After diabetes was induced (fasting glucose level ≥ 250 mg/dL), the mice were treated with tocopherol-stripped corn oil or GT-supplemented (35 mg/kg) corn oil, respectively, by gavage for 2 weeks.
RESULTS
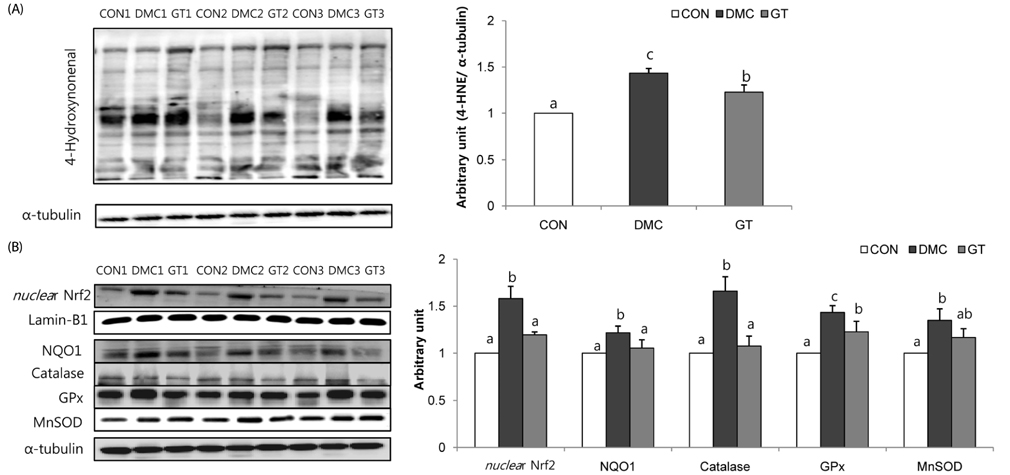
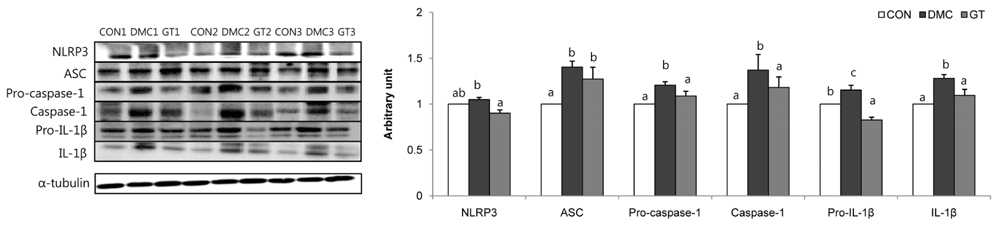
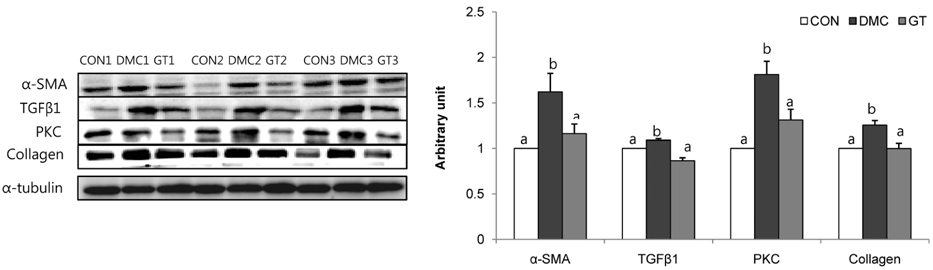
GT supplementation reduced fasting blood glucose levels in diabetic mice relative to non-treated diabetic mice. Moreover, GT supplementation ameliorated hyperglycemia-induced hepatic damage by regulation of NOD-like receptor protein 3 (NLRP3)-inflammasome associated inflammation represented by NLRP3, apoptosis-associated speck-like protein containing a caspase-recruitment domain, caspase-1, nuclear factor-κB pathway as well as oxidative stress demonstrated by nuclear factor erythroid 2-related factor 2, NAD(P)H dehydrogenase quinone 1, catalase and glutathione-dependent peroxidase in diabetic mice.
CONCLUSION
The findings suggested that GT supplementation ameliorated hepatic damage by attenuating inflammation and oxidative stress in alloxan-induced diabetic mice. Taken together, GT could be a beneficial nutrient that can ameliorate inflammatory responses associated with NLRP3 inflammasome in hyperglycemia-induced hepatic damage.
Keyword
MeSH Terms
-
Alloxan
Animals
Blood Glucose
Catalase
Corn Oil
Diet
Fasting
gamma-Tocopherol*
Glucose
Hyperglycemia
Inflammasomes*
Inflammation*
Injections, Intraperitoneal
Liver
Mice*
Mice, Inbred ICR
Oxidative Stress
Oxidoreductases
Peroxidase
Alloxan
Blood Glucose
Catalase
Corn Oil
Glucose
Inflammasomes
Oxidoreductases
Peroxidase
gamma-Tocopherol
Figure
Reference
-
1. Pagano E, De Rosa M, Rossi E, Cinconze E, Marchesini G, Miccoli R, Vaccaro O, Bonora E, Bruno G, Observatory AD. The relative burden of diabetes complications on healthcare costs: the population-based CINECA-SID ARNO Diabetes Observatory. Nutr Metab Cardiovasc Dis. 2016; 26:944–950.
Article2. Regnell SE, Lernmark Å. Hepatic steatosis in type 1 diabetes. Rev Diabet Stud. 2011; 8:454–467.
Article3. Käräjämäki AJ, Bloigu R, Kauma H, Kesäniemi YA, Koivurova OP, Perkiömäki J, Huikuri H, Ukkola O. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metabolism. 2017; 66:55–63.
Article4. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003; 17:24–38.
Article5. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016; 73:3221–3247.
Article6. Mohamed J, Nazratun Nafizah AH, Zariyantey AH, Budin SB. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016; 16:e132–e141.
Article7. Hu C, Ding H, Li Y, Pearson JA, Zhang X, Flavell RA, Wong FS, Wen L. NLRP3 deficiency protects from type 1 diabetes through the regulation of chemotaxis into the pancreatic islets. Proc Natl Acad Sci U S A. 2015; 112:11318–11323.
Article8. Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T, Rouis M. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015; 4:296–307.
Article9. Kang HH, Kim IK, Lee HI, Joo H, Lim JU, Lee J, Lee SH, Moon HS. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem Biophys Res Commun. 2017; 490:349–355.
Article10. Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013; 25:1939–1948.
Article11. Cao Y, Jiang X, Ma H, Wang Y, Xue P, Liu Y. SIRT1 and insulin resistance. J Diabetes Complications. 2016; 30:178–183.
Article12. Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in liver fibrosis. Semin Liver Dis. 2017; 37:119–127.
Article13. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017; 127:55–64.
Article14. Li J, Cordero P, Nguyen V, Oben JA. The role of vitamins in the pathogenesis of non-alcoholic fatty liver disease. Integr Med Insights. 2016; 11:19–25.
Article15. Lee H, Lim Y. Tocotrienol-rich fraction supplementation reduces hyperglycemia-induced skeletal muscle damage through regulation of insulin signaling and oxidative stress in type 2 diabetic mice. J Nutr Biochem. 2018; 57:77–85.
Article16. Smolarek AK, Suh N. Chemopreventive activity of vitamin E in breast cancer: a focus on γ- and δ-tocopherol. Nutrients. 2011; 3:962–986.
Article17. Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003; 17:816–822.
Article18. Shin H, Eo H, Lim Y. Similarities and differences between alpha-tocopherol and gamma-tocopherol in amelioration of inflammation, oxidative stress and pre-fibrosis in hyperglycemia induced acute kidney inflammation. Nutr Res Pract. 2016; 10:33–41.
Article19. Shin J, Yang SJ, Lim Y. Gamma-tocopherol supplementation ameliorated hyper-inflammatory response during the early cutaneous wound healing in alloxan-induced diabetic mice. Exp Biol Med (Maywood). 2017; 242:505–515.
Article20. Peh HY, Tan WS, Liao W, Wong WS. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol Ther. 2016; 162:152–169.
Article21. Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003; 64:978–991.
Article22. Uusitalo L, Nevalainen J, Niinistö S, Alfthan G, Sundvall J, Korhonen T, Kenward MG, Oja H, Veijola R, Simell O, Ilonen J, Knip M, Virtanen SM. Serum alpha- and gamma-tocopherol concentrations and risk of advanced beta cell autoimmunity in children with HLA-conferred susceptibility to type 1 diabetes mellitus. Diabetologia. 2008; 51:773–780.
Article23. Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000; 97:11494–11499.
Article24. Roldi LP, Pereira RV, Tronchini EA, Rizo GV, Scoaris CR, Zanoni JN, Natali MR. Vitamin E (alpha-tocopherol) supplementation in diabetic rats: effects on the proximal colon. BMC Gastroenterol. 2009; 9:88.
Article25. Kim GH, Chung JW, Lee JH, Ok KS, Jang ES, Kim J, Shin CM, Park YS, Hwang JH, Jeong SH, Kim N, Lee DH, Kim JW. Effect of vitamin E in nonalcoholic fatty liver disease with metabolic syndrome: A propensity score-matched cohort study. Clin Mol Hepatol. 2015; 21:379–386.
Article26. Agca CA, Tuzcu M, Hayirli A, Sahin K. Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem Toxicol. 2014; 71:116–121.
Article27. Gupte AA, Lyon CJ, Hsueh WA. Nuclear factor (erythroid-derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steatohepatitis. Curr Diab Rep. 2013; 13:362–371.
Article28. Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014; 5:352.
Article29. Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002; 106:2067–2072.
Article30. Li X, Gao Y, Xu H, Hou J, Gao P. Diabetes mellitus is a significant risk factor for the development of liver cirrhosis in chronic hepatitis C patients. Sci Rep. 2017; 7:9087.
Article31. Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999; 19:350–364.32. Ingaramo PI, Ronco MT, Francés DE, Monti JA, Pisani GB, Ceballos MP, Galleano M, Carrillo MC, Carnovale CE. Tumor necrosis factor alpha pathways develops liver apoptosis in type 1 diabetes mellitus. Mol Immunol. 2011; 48:1397–1407.
Article33. Carnovale CE, Ronco MT. Role of nitric oxide in liver regeneration. Ann Hepatol. 2012; 11:636–647.
Article34. Zheng QY, Cao ZH, Hu XB, Li GQ, Dong SF, Xu GL, Zhang KQ. LIGHT/IFN-γ triggers β cells apoptosis via NF-κB/Bcl2-dependent mitochondrial pathway. J Cell Mol Med. 2016; 20:1861–1871.
Article35. Boumela I, Assou S, Aouacheria A, Haouzi D, Dechaud H, De Vos J, Handyside A, Hamamah S. Involvement of BCL2 family members in the regulation of human oocyte and early embryo survival and death: gene expression and beyond. Reproduction. 2011; 141:549–561.
Article36. Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010; 31:533–542.
Article37. Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014; 59:898–910.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Similarities and differences between alpha-tocopherol and gamma-tocopherol in amelioration of inflammation, oxidative stress and pre-fibrosis in hyperglycemia induced acute kidney inflammation
- Cobalt Chloride-induced Hypoxia Ameliorates NLRP3-Mediated Caspase-1 Activation in Mixed Glial Cultures
- The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout
- Loganin Prevents Hepatic Steatosis by Blocking NLRP3 Inflammasome Activation
- FoxO6-Mediated TXNIP Induces Lipid Accumulation in the Liver through NLRP3 Inflammasome Activation