Nutr Res Pract.
2019 Oct;13(5):367-376. 10.4162/nrp.2019.13.5.367.
Anti-diabetic effects of blue honeyberry on high-fed-diet-induced type II diabetic mouse
- Affiliations
-
- 1Department of Food and Nutrition, College of BioNano Technology, Gachon University, 1342 Seongnamdaero, Sujeong-gu, Seongnam, Gyeonggi 13120, Republic of Korea. skysea@gachon.ac.kr
- 2Aribio Co. Ltd., #2-301, Pangyo Seven Venture Valley, Gyeonggi 13487, Republic of Korea.
- 3Department of Anatomy and Histology, College of Korean Medicine, Daegu Haany University, Gyeongbuk 38610, Republic of Korea.
- 4Major in Food Biotechnology, Division of Bioindustry, College of Medical and Life Sciences, Silla University, 140, Baegyang-daero 700beon-gil, Sasang-gu, Busan 46958, Republic of Korea. jsc1008@silla.ac.kr
- KMID: 2464115
- DOI: http://doi.org/10.4162/nrp.2019.13.5.367
Abstract
- BACKGROUND
/OBJECTIVE: The blue honeysuckle berry (Lonicera caerulea var. edulis L.) is a small deciduous shrub belonging to the Caprifoliaceae family that is native to Russia, China, Japan, and Korea. The berry of this shrub is edible, sweet and juicy and is commonly known as the blue honeyberry (BHB). This study examined the anti-diabetic potential of BHB on high-fat-diet-induced mild diabetic mice. The hypoglycemic, and nephroprotective effects of the 12-week oral administration of blue honeyberry extract were analyzed.
MATERIALS/METHODS
The hypoglycemic effects were based on the observed changes in insulin, blood glucose, and glycated hemoglobin (HbA1c). Furthermore, the changes in the weight of the pancreas, including its histopathology and immunohistochemical investigation were also performed. Moreover, the nephroprotective effects were analyzed by observing the changes in kidney weight, its histopathology, blood urea nitrogen (BUN), and serum creatinine levels.
RESULTS
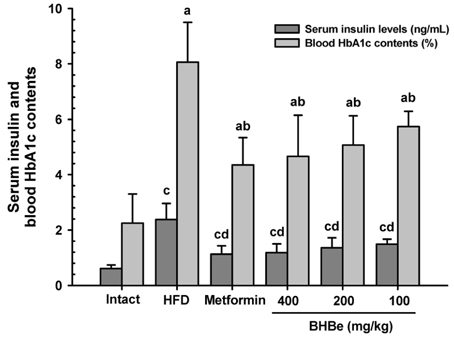
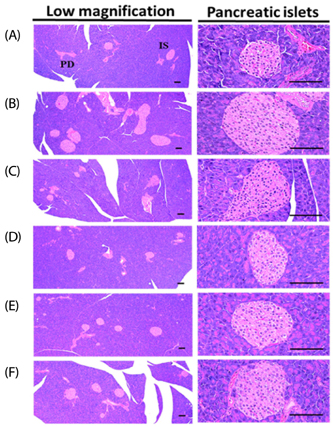
The results showed that the high-fat diet (HFD)-induced control mice showed a noticeable increase in blood glucose, insulin, HbA1c, BUN, and creatinine levels. Furthermore, growth was observed in lipid droplet deposition related to the degenerative lesions in the vacuolated renal tubules with the evident enlargement and hyperplasia of the pancreatic islets. In addition, in the endocrine pancreas, there was an increase in the insulin-and glucagon-producing cells, as well as in the insulin/glucagon cell ratios. On the other hand, compared to the HFD-treated mice group, all these diabetic and related complications were ameliorated significantly in a dose-dependent manner after 84 days of the continuous oral administration of BHBe at 400, 200 and 100 mg/kg, and a dramatic resettlement in the hepatic glucose-regulating enzyme activities was observed.
CONCLUSIONS
By assessing the key parameters for T2DM, the present study showed that the BHBe could act as a potential herbal agent to cure diabetes (type II) and associated ailments in HFD-induced mice.
MeSH Terms
-
Administration, Oral
Animals
Blood Glucose
Blood Urea Nitrogen
Caprifoliaceae
China
Creatinine
Diet, High-Fat
Fruit
Glucagon
Hand
Hemoglobin A, Glycosylated
Humans
Hyperplasia
Hypoglycemic Agents
Insulin
Islets of Langerhans
Japan
Kidney
Korea
Lipid Droplets
Lonicera
Metformin
Mice*
Pancreas
Russia
Blood Glucose
Creatinine
Glucagon
Hypoglycemic Agents
Insulin
Metformin
Figure
Reference
-
1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018; 138:271–281.
Article2. Sami W, Ansari T, Butt NS, Hamid MRA. Effect of diet on type 2 diabetes mellitus: a review. Int J Health Sci (Qassim). 2017; 11:65–71.3. Enk J, Mandelboim O. The role of natural cytotoxicity receptors in various pathologies: emphasis on type I diabetes. Front Immunol. 2014; 5:4.
Article4. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012; 2012:918267.
Article5. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014; 383:1068–1083.
Article6. Wright JJ, Tylee TS. Pharmacologic therapy of type 2 diabetes. Med Clin North Am. 2016; 100:647–663.
Article7. Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017; 13:3–10.
Article8. Lindström J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinänen-Kiukaanniemi S, Uusitupa M, Tuomilehto J. Finnish Diabetes Prevention Study (DPS). Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013; 56:284–293.
Article9. Shah P, Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin Drug Saf. 2010; 9:347–354.
Article10. Kang SJ, Lee JE, Lee EK, Jung DH, Song CH, Park SJ, Choi SH, Han CH, Ku SK, Lee YJ. Fermentation with Aquilariae lignum enhances the anti-diabetic activity of green tea in type II diabetic db/db mouse. Nutrients. 2014; 6:3536–3571.
Article11. Mottillo EP, Desjardins EM, Fritzen AM, Zou VZ, Crane JD, Yabut JM, Kiens B, Erion DM, Lanba A, Granneman JG, Talukdar S, Steinberg GR. FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol Metab. 2017; 6:471–481.
Article12. Ma A, Wang J, Yang L, An Y, Zhu H. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE−/− mice. J Lipid Res. 2017; 58:1536–1547.
Article13. Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: Post hoc analysis of a randomized controlled 4.3year trial. J Diabetes Complications. 2018; 32:171–178.
Article14. Zdilla MJ. Metformin with either histamine H2-receptor antagonists or proton pump inhibitors: A polypharmacy recipe for neuropathy via vitamin B12 depletion. Clin Diabetes. 2015; 33:90–95.
Article15. Khurana R, Malik IS. Metformin: safety in cardiac patients. Heart. 2010; 96:99–102.
Article16. Chaovanalikit A, Thompson MM, Wrolstad RE. Characterization and quantification of anthocyanins and polyphenolics in blue honeysuckle (Lonicera caerulea L.). J Agric Food Chem. 2004; 52:848–852.
Article17. Svarcova I, Heinrich J, Valentova K. Berry fruits as a source of biologically active compounds: the case of Lonicera caerulea. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007; 151:163–174.
Article18. Liu S, Xu Q, Li X, Wang Y, Zhu J, Ning C, Chang X, Meng X. Effects of high hydrostatic pressure on physicochemical properties, enzymes activity, and antioxidant capacities of anthocyanins extracts of wild Lonicera caerulea berry. Innov Food Sci Emerg Technol. 2016; 36:48–58.
Article19. Oszmiański J, Wojdyło A, Lachowicz S. Effect of dried powder preparation process on polyphenolic content and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L. var. kamtschatica). LWT-Food Sci Technol. 2016; 67:214–222.
Article20. Becker R, Pączkowski C, Szakiel A. Triterpenoid profile of fruit and leaf cuticular waxes of edible honeysuckle Lonicera caerulea var. kamtschatica. Acta Soc Bot Pol. 2017; 86:3539.
Article21. Chen L, Xin X, Yuan Q, Su D, Liu W. Phytochemical properties and antioxidant capacities of various colored berries. J Sci Food Agric. 2014; 94:180–188.
Article22. Zhao H, Wang Z, Ma F, Yang X, Cheng C, Yao L. Protective effect of anthocyanin from Lonicera caerulea var. edulis on radiation-induced damage in mice. Int J Mol Sci. 2012; 13:11773–11782.
Article23. Palíková I, Valentová K, Oborná I, Ulrichová J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J Agric Food Chem. 2009; 57:6584–6589.
Article24. Vostálová J, Galandáková A, Palíková I, Ulrichová J, Doležal D, Lichnovská R, Vrbková J, Rajnochová SA. Lonicera caerulea fruits reduce UVA-induced damage in hairless mice. J Photochem Photobiol B. 2013; 128:1–11.25. Wu D, Zheng N, Qi K, Cheng H, Sun Z, Gao B, Zhang Y, Pang W, Huangfu C, Ji S, Xue M, Ji A, Li Y. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med Gas Res. 2015; 5:1.
Article26. Park SI, Lee YJ, Choi SH, Park SJ, Song CH, Ku SK. Therapeutic effects of blue honeysuckle on lesions of hyperthyroidism in rats. Am J Chin Med. 2016; 44:1441–1456.
Article27. Jurgoński A, Juśkiewicz J, Zduńczyk Z. An anthocyanin-rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition. 2013; 29:898–902.
Article28. Wu S, He X, Wu X, Qin S, He J, Zhang S, Hou DX. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: crosstalk of anti-inflammatory and antioxidant effects. J Funct Foods. 2015; 17:514–523.
Article29. Zhao J, Lin Y, Zhao Y, Wang Y, Ning C, Ma Y, Meng X. Polyphenol-rich blue honeysuckle extract alleviates silica particle-induced inflammatory responses and macrophage apoptosis via NRF2/HO-1 and MAPK signaling. J Funct Foods. 2018; 46:463–474.
Article30. Liu S, You L, Zhao Y, Chang X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res Int. 2018; 107:73–83.
Article31. Liu S, Wu Z, Guo S, Meng X, Chang X. Polyphenol-rich extract from wild Lonicera caerulea berry reduces cholesterol accumulation by mediating the expression of hepatic miR-33 and miR-122, HMGCR, and CYP7A1 in rats. J Funct Foods. 2018; 40:648–658.
Article32. Liu M, Tan J, He Z, He X, Hou DX, He J, Wu S. Inhibitory effect of blue honeysuckle extract on high-fat-diet-induced fatty liver in mice. Anim Nutr. 2018; 4:288–293.
Article33. Lee HS, Chang JH, Ku SK. An immunohistochemical study of the pancreatic endocrine cells of the ddN mouse. Folia Histochem Cytobiol. 2010; 48:387–393.
Article34. Lee JE, Kang SJ, Choi SH, Song CH, Lee YJ, Ku SK. Fermentation of green tea with 2% Aquilariae lignum increases the anti-diabetic activity of green tea aqueous extracts in the high fat-fed mouse. Nutrients. 2015; 7:9046–9078.
Article35. Lee HS, Chang JH, Ku SK. An immunohistochemical study of the pancreatic endocrine cells of the ddN mouse. Folia Histochem Cytobiol. 2010; 48:387–393.
Article36. Levene A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin Otolaryngol Allied Sci. 1981; 6:145–151.
Article37. Ludbrook J. Update: microcomputer statistics packages. A personal view. Clin Exp Pharmacol Physiol. 1997; 24:294–296.
Article38. Choi JS, Kim JW, Park JB, Pyo SE, Hong YK, Ku SK, Kim MR. Blood glycemia-modulating effects of melanian snail protein hydrolysates in mice with type II diabetes. Int J Mol Med. 2017; 39:1437–1451.
Article39. Jurikova T, Rop O, Mlcek J, Sochor J, Balla S, Szekeres L, Hegedusova A, Hubalek J, Adam V, Kizek R. Phenolic profile of edible honeysuckle berries (Genus Lonicera) and their biological effects. Molecules. 2011; 17:61–79.
Article40. Rupasinghe HPV, Arumuggam N, Amararathna M, De Silva ABKH. The potential health benefits of haskap (Lonicera caerulea L.): role of cyanidin-3-O-glucoside. J Funct Foods. 2018; 44:24–39.
Article41. Sancho RAS, Pastore GM. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res Int. 2012; 46:378–386.
Article42. Podsędek A, Majewska I, Redzynia M, Sosnowska D, Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J Agric Food Chem. 2014; 62:4610–4617.
Article43. Tan Y, Kim J, Cheng J, Ong M, Lao WG, Jin XL, Lin YG, Xiao L, Zhu XQ, Qu XQ. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J Gastroenterol. 2017; 23:3805–3814.
Article44. Kim UH, Yoon JH, Li H, Kang JH, Ji HS, Park KH, Shin DH, Park HY, Jeong TS. Pterocarpan-enriched soy leaf extract ameliorates insulin sensitivity and pancreatic β-cell proliferation in type 2 diabetic mice. Molecules. 2014; 19:18493–18510.
Article45. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004; 279:32345–32353.
Article46. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007; 87:507–520.
Article47. Chen H, Qu Z, Fu L, Dong P, Zhang X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J Food Sci. 2009; 74:C469–C474.
Article48. Ku SK, Sung SH, Choung JJ, Choi JS, Shin YK, Kim JW. Anti-obesity and anti-diabetic effects of a standardized potato extract in ob/ob mice. Exp Ther Med. 2016; 12:354–364.
Article49. Lo HY, Hsiang CY, Li TC, Li CC, Huang HC, Chen JC, Ho TY. A novel glycated hemoglobin A1c-lowering traditional Chinese medicinal formula, identified by translational medicine study. PLoS One. 2014; 9:e104650.
Article50. Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, Imperatore G, Williams DE, Albright AL. A1C level and future risk of diabetes: a systematic review. Diabetes Care. 2010; 33:1665–1673.
Article51. Kim CM, Yi SJ, Cho IJ, Ku SK. Red-koji fermented red ginseng ameliorates high fat diet-induced metabolic disorders in mice. Nutrients. 2013; 5:4316–4332.
Article52. Chung SI, Rico CW, Kang MY. Comparative study on the hypoglycemic and antioxidative effects of fermented paste (doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients. 2014; 6:4610–4624.
Article53. Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007; 117:246–257.
Article54. Noriega-López L, Tovar AR, Gonzalez-Granillo M, Hernández-Pando R, Escalante B, Santillán-Doherty P, Torres N. Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J Biol Chem. 2007; 282:20657–20666.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Polygonatum odoratum on In vivo Insulin Activity in Streptozotocin-Induced Diabetic Rats
- Alterations in the blood glucose, serum lipids and renal oxidative stress in diabetic rats by supplementation of onion (Allium cepa. Linn)
- Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice
- Effects of soybean isoflavone extract on the plasma lipid profiles and antioxidant enzyme activity in streptozotocin-induced diabetic rats
- Clinical Analysis of Diabetic Retinopathy According to the Type of Diabetes Mellitus