J Liver Cancer.
2019 Sep;19(2):159-164. 10.17998/jlc.19.2.159.
Hepatocellular Carcinoma with Segmental Portal Vein Invasion Exhibiting a Complete Response after Transarterial Radioembolization
- Affiliations
-
- 1Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea. ydoctor2@hanmail.net
- 2Department of Internal Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2463618
- DOI: http://doi.org/10.17998/jlc.19.2.159
Abstract
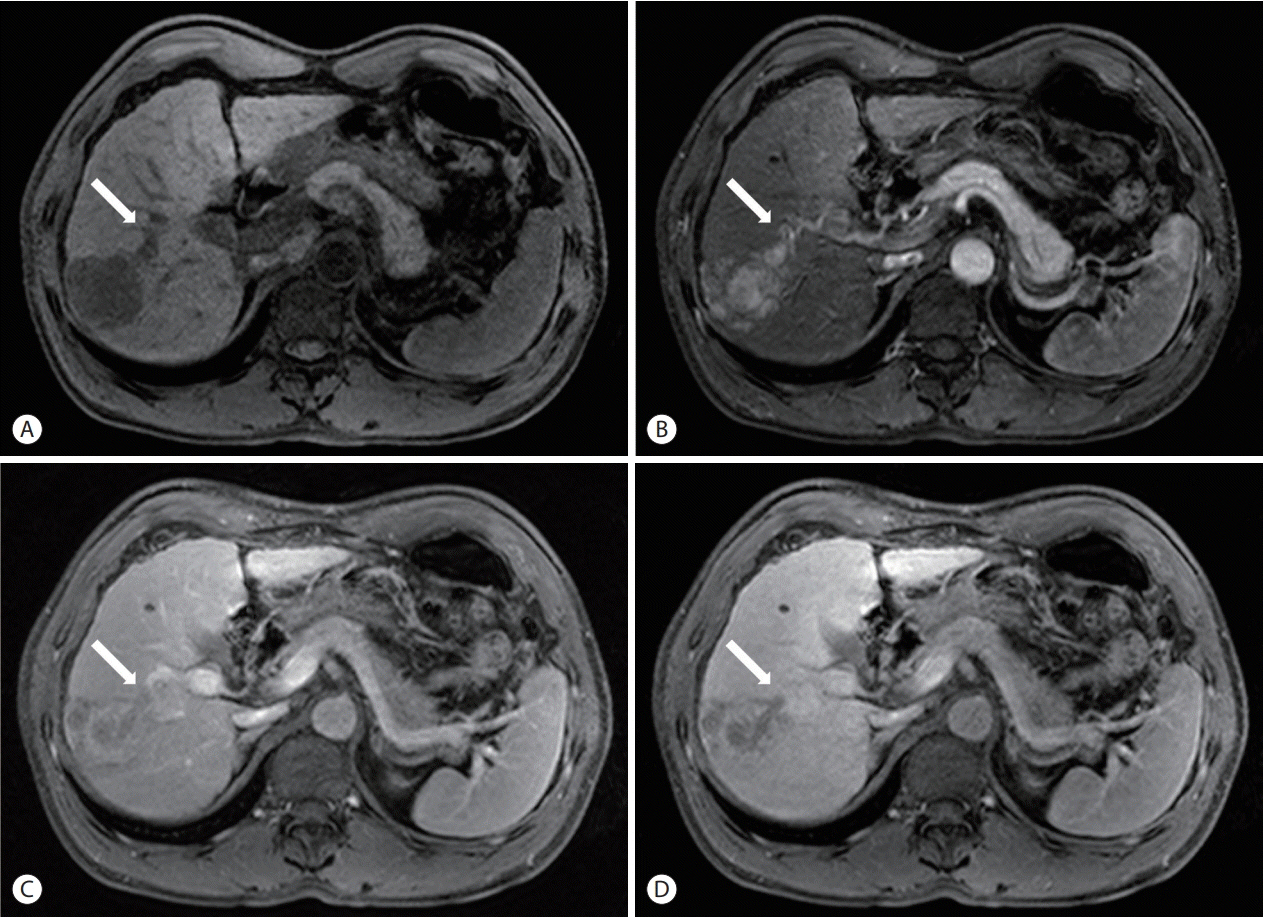
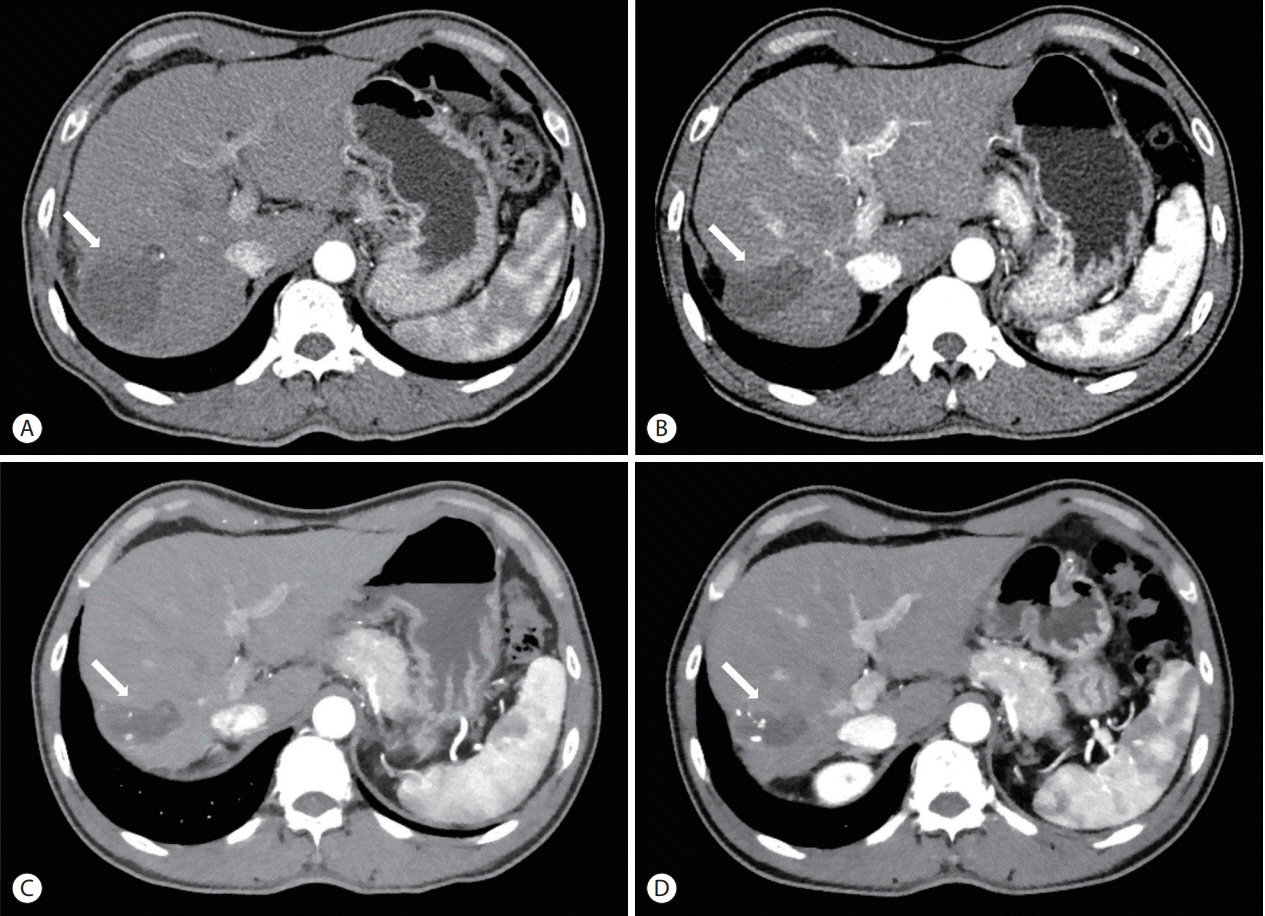
- The treatment options available for patients with hepatocellular carcinoma (HCC) with portal vein invasion (PVI) include sorafenib, transarterial radioembolization (TARE), radiation therapy (RT), transarterial chemoembolization with RT, and proton beam irradiation. Herein, we present a case of HCC with segmental PVI that was managed via TARE. The patient had a 4 cm HCC that invaded the segment VIII portal vein branch without extrahepatic spread. Liver function was Child-Pugh grade A, and performance status was good. TARE was performed without any adverse events, and a radiological complete response (CR) was achieved. Thereafter, the patient was followed-up every 3-6 months without any further treatment, and the CR was maintained for >3 years. Therefore, TARE may be a useful alternative therapeutic option for patients with HCC exhibiting segmental PVI.
Keyword
MeSH Terms
Figure
Reference
-
1. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.2. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017; 67:999–1008.3. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.4. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.5. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.6. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015; 62:1187–1195.7. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.8. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.9. Kim DY, Han KH. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int. 2016; 10:883–892.10. Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016; 33:699–714.11. Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis. 2014; 34:435–443.12. Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013; 36:714–723.13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.14. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 noninferiority trial. Lancet. 2018; 391:1163–1173.15. Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010; 17:2073–2080.16. Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005; 140:450–457. ;discussion 457-458.17. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018; 68:1429–1440.18. Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017; 18:1624–1636.19. Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018; 36:1913–1921.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- A Case of Successful Hepatic Resection after Insufficient Response to Transarterial Chemoembolization and Radiation Therapy in Hepatocellular Carcinoma with Portal Vein Invasion
- Role of Yttrium-90 Radioembolization in the Management of Hepatocellular Carcinoma
- Long-term survival after CCRT and HAIC followed by ALPPS for hepatocellular carcinoma with portal vein invasion: a case report
- Radioembolization for the treatment of hepatocellular carcinoma