Ann Surg Treat Res.
2019 Nov;97(5):230-238. 10.4174/astr.2019.97.5.230.
Inhibition of tamoxifen's therapeutic effects by emodin in estrogen receptor-positive breast cancer cell lines
- Affiliations
-
- 1Department of Surgery, Bundang Jesang General Hospital, Seongnam, Korea.
- 2Clinical Science, Department of Medicine, The Graduate School of Konkuk University, Seoul, Korea.
- 3Division of Radiation Cancer Research, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Ewha Womans University Mokdong Hospital/Cancer Center for Women, Breast and Thyroid Cancer Center, Seoul, Korea.
- 5Department of Surgery, Kyung Hee University School of Medicine, Seoul, Korea.
- 6Department of Surgery, Konkuk University School of Medicine, Seoul, Korea. ks2002p@hanmail.net
- 7Research Institute of Medical Science, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2461898
- DOI: http://doi.org/10.4174/astr.2019.97.5.230
Abstract
- PURPOSE
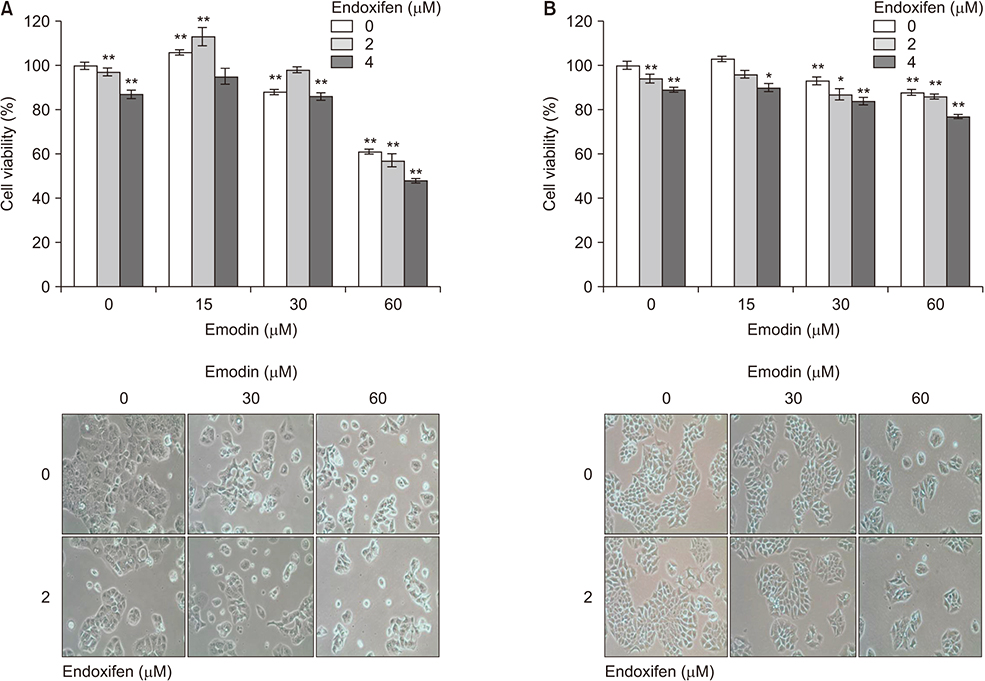
This study was aimed to investigate the combination effect of endoxifen and emodin on estrogen receptor (ER) positive breast cancer cell lines and to explain the mechanism of the combination effect.
METHODS
We conducted this study on MCF-7 (ER+/human epidermal growth factor receptor-2 [HER2]−), T47D (ER+/HER2−), ZR-75-1 (ER+/HER2+), and BT474 (ER+/HER2+) cell lines, which confirmed combination effect of endoxifen and emodin. Optimal concentrations for combination were determined to study the effects on proliferation of MCF-7 and ZR-75-1 cells. Analysis of the combination effect was carried out in the CompuSyn software. The combination of downstream mechanisms, and combined effects of other similar compounds were tested on the MCF-7 and ZR 75-1 cell lines. Protein expression was confirmed by western blot.
RESULTS
The combination of endoxifen and emodin had antagonistic effects on MCF-7 and ZR-75-1cell lines (combination index > 1). We validated the antagonistic effect in T47D and BT474 cell lines. During the combined treatment, the results showed elevated amounts of cyclin D1 and phosphorylated extracellular signal-regulated kinase (pERK). Analysis of drug interactions showed antagonistic effect between endoxifen and chemical compounds similar to emodin, such as chrysophanol or rhein, in MCF-7 and ZR-75-1 cells.
CONCLUSION
Addition of emodin attenuated tamoxifen's treatment effect via cyclin D1 and pERK up-regulation in ER-positive breast cancer cell lines.
Keyword
MeSH Terms
Figure
Reference
-
1. Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996–2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400.
Article2. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998; 339:1609–1618.
Article3. Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998; 351:1451–1467.4. Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014; 32:2255–2269.
Article5. Aiello Bowles EJ, Boudreau DM, Chubak J, Yu O, Fujii M, Chestnut J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract. 2012; 8:e149–e157.
Article6. Jiao Y, Zuo Y. Ultrasonic extraction and HPLC determination of anthraquinones, aloe-emodine, emodine, rheine, chrysophanol and physcione, in roots of Polygoni multiflori. Phytochem Anal. 2009; 20:272–278.7. Lin SY, Lai WW, Ho CC, Yu FS, Chen GW, Yang JS, et al. Emodin induces apoptosis of human tongue squamous cancer SCC-4 cells through reactive oxygen species and mitochondria-dependent pathways. Anticancer Res. 2009; 29:327–335.8. Basu S, Ghosh A, Hazra B. Evaluation of the antibacterial activity of Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn.: isolation of emodin and physcion as active antibacterial agents. Phytother Res. 2005; 19:888–894.9. Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013; 341:139–149.
Article10. Wang XD, Gu LQ, Wu JY. Apoptosis-inducing activity of new pyrazole emodin derivatives in human hepatocellular carcinoma HepG2 cells. Biol Pharm Bull. 2007; 30:1113–1116.
Article11. Meng G, Liu Y, Lou C, Yang H. Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-κB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br J Pharmacol. 2010; 161:1628–1644.
Article12. Wei WT, Lin SZ, Liu DL, Wang ZH. The distinct mechanisms of the antitumor activity of emodin in different types of cancer (Review). Oncol Rep. 2013; 30:2555–2562.
Article13. Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011; 13:215.
Article14. Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006; 318:503–512.
Article15. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006; 58:621–681.
Article16. Chung YS, Cho S, Ryou HJ, Jee HG, Choi JY, Yoon K, et al. Is there a treatment advantage when paclitaxel and lovastatin are combined to dose anaplastic thyroid carcinoma cell lines? Thyroid. 2011; 21:735–744.
Article17. Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997; 57:3071–3078.18. Burdall SE, Hanby AM, Lansdown MR, Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res. 2003; 5:89–95.
Article19. Sartorius CA, Groshong SD, Miller LA, Powell RL, Tung L, Takimoto GS, et al. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994; 54:3868–3877.20. Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006; 10:515–527.
Article21. Pontano LL, Diehl JA. Speeding through cell cycle roadblocks: nuclear cyclin D1-dependent kinase and neoplastic transformation. Cell Div. 2008; 3:12.
Article22. Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006; 125:1137–1149.
Article23. Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res. 2007; 67:7256–7265.
Article24. Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013; 39:935–946.
Article25. Mohammend MM. Structure antimutagenicity relationship of anthraquinones. Nat Prod Chem Res. 2016; 4:1000228.
Article26. Jones JL, Daley BJ, Enderson BL, Zhou JR, Karlstad MD. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am Surg. 2002; 68:575–577.27. Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002; 62:2474–2477.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anticancer effect of tamoxifen in oral cancer cell
- The Effect of Tamoxifen of the Estrogen Receptor cDNA-Iipofected MDA-MB-231 Human Breast Cancer Cells
- Restoration of Hormone Dependency in Estrogen Receptor - Lipofected MDA-MB-231 Human Breast Cancer Cells
- Tamoxifen Resistance and Crosstalk of Signal Transduction in Breast Cancer
- Cell Division Cycle Associated 8 Is a Key Regulator of Tamoxifen Resistance in Breast Cancer