Allergy Asthma Respir Dis.
2019 Oct;7(4):212-217. 10.4168/aard.2019.7.4.212.
A pediatric case of eosinophilic granulomatosis with polyangiitis accompanied by heart failure mimicking an asthma attack
- Affiliations
-
- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. dongins0@snu.ac.kr
- KMID: 2461399
- DOI: http://doi.org/10.4168/aard.2019.7.4.212
Abstract
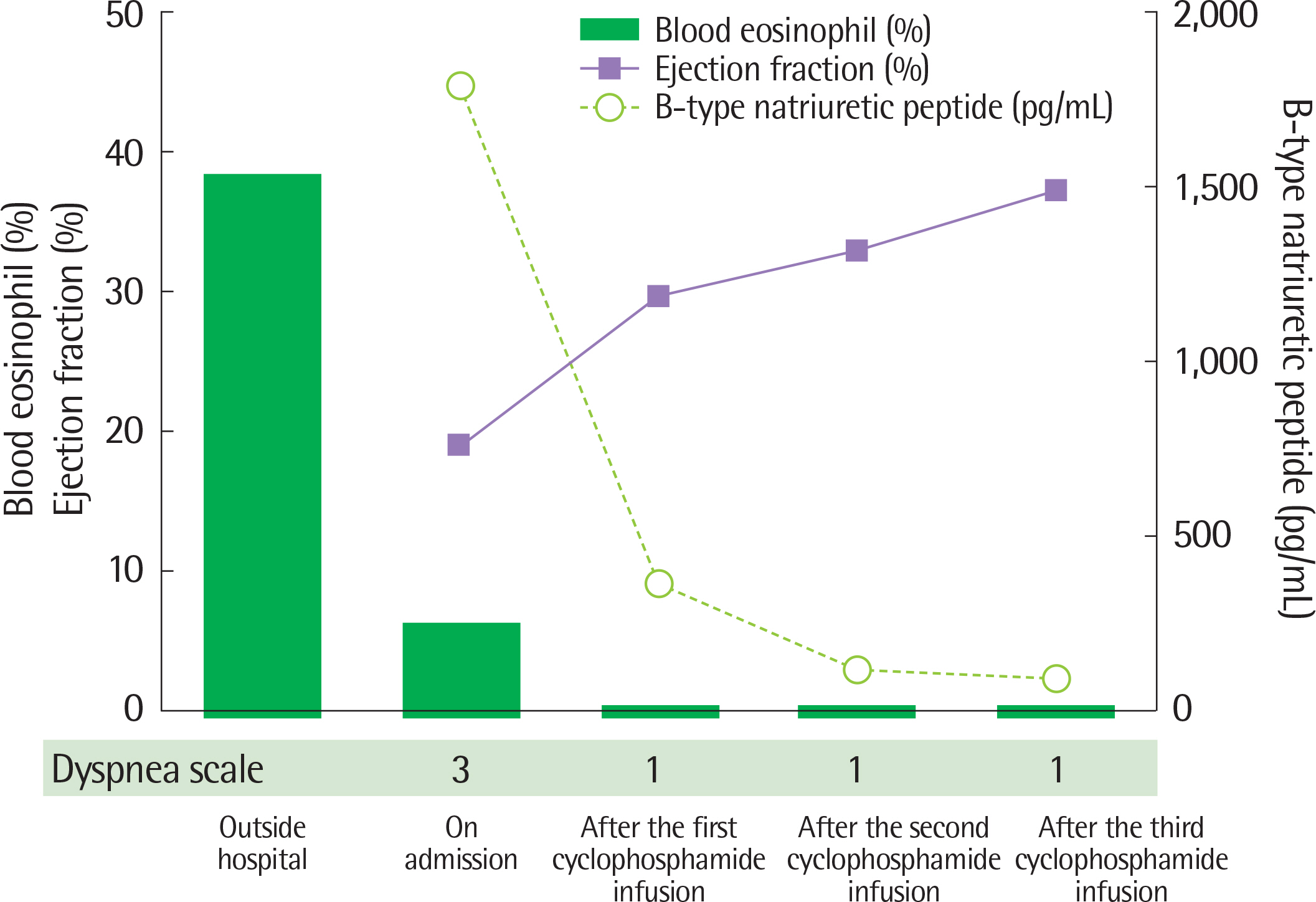
- Eosinophilic granulomatosis with polyangiitis (EGPA, also known as the Churg-Strauss syndrome) is a disorder characterized by asthma, peripheral eosinophilia and systemic vasculitis. It rarely occurs in children, so that physicians may frequently mistake it for a simple uncontrolled asthma. Since a subsequent cardiac involvement is critical for the prognosis, it is important to suspect EGPA in children with severe, uncontrolled asthma. The cardiac manifestations in EGPA are variable from asymptomatic electrocardiogram abnormalities to pericarditis with pericardial effusion, myocarditis with cardiomyopathy, heart failure, and sudden cardiac death. Although delayed treatment may lead to fatal cardiac complications in EGPA, adequate immune suppression can reverse cardiac impairment. We report a 14-year-old girl with persistent asthma refractory to steroids who was eventually diagnosed with an anti-neutrophil cytoplasmic antibody-negative EGPA.
MeSH Terms
Figure
Reference
-
References
1. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periar-teritis nodosa. Am J Pathol. 1951; 27:277–301.2. Sinico RA, Bottero P. Churg-Strauss angiitis. Best Pract Res Clin Rheumatol. 2009; 23:355–66.
Article3. Yano T, Ishimura S, Furukawa T, Koyama M, Tanaka M, Shimoshige S, et al. Cardiac tamponade leading to the diagnosis of eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a case report and review of the literature. Heart Vessels. 2015; 30:841–4.
Article4. Seeliger B, Sznajd J, Robson JC, Judge A, Craven A, Grayson PC, et al. Are the 1990 American College of Rheumatology vasculitis classification criteria still valid? Rheumatology (Oxford). 2017; 56:1154–61.
Article5. Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and longterm followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013; 65:270–81.
Article6. Mahr A, Moosig F, Neumann T, Szczeklik W, Taillé C, Vaglio A, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): evolutions in classification, etiopathogenesis, assessment and management. Curr Opin Rheumatol. 2014; 26:16–23.7. Gendelman S, Zeft A, Spalding SJ. Childhood-onset eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome): a contemporary singlecenter cohort. J Rheumatol. 2013; 40:929–35.
Article8. Eleftheriou D, Gale H, Pilkington C, Fenton M, Sebire NJ, Brogan PA. Eosinophilic granulomatosis with polyangiitis in childhood: retrospective experience from a tertiary referral centre in the UK. Rheumatology (Oxford). 2016; 55:1263–72.
Article9. Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and longterm follow-up of 96 patients. Medicine (Baltimore). 1999; 78:26–37.
Article10. Jung SH, Kim KH, Nam SM, Park HC, Chu HK, Whang IS, et al. A case of Churg-Strauss syndrome with manifestations of esophageal ulcer, acute acalculous cholecystitis and ischemic colitis. Korean J Intern Med. 1993; 45:369–75.11. Lee WJ, Hwang JW, Kim E, Yune S, Ha JM, Yoon N, et al. Churg-Strauss syndrome presenting as acute acalculous cholecystitis. Allergy Asthma Respir Dis. 2013; 1:388–90.
Article12. Choi JY, Kim JE, Choi IY, Lee JH, Kim JH, Shin C, et al. Churg-Strauss syndrome that presented with mediastinal lymphadenopathy and calcu-lous cholecystitis. Korean J Intern Med. 2016; 31:179–83.
Article13. Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Ruoppolo G, et al. Churg-Strauss syndrome. Autoimmun Rev. 2015; 14:341–8.
Article14. Guillevin L, Lhote F, Gayraud M, Cohen P, Jarrousse B, Lortholary O, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine (Baltimore). 1996; 75:17–28.
Article15. Hazebroek MR, Kemna MJ, Schalla S, Sanders-van Wijk S, Gerretsen SC, Dennert R, et al. Prevalence and prognostic relevance of cardiac involvement in ANCA-associated vasculitis: eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int J Cardiol. 2015; 199:170–9.
Article16. Park S, Kim T, Kim HJ, Shin B, Park SY, Kwon HS, et al. Heart transplantation in a patient with eosinophilic granulomatosis with polyangiitis known as Churg-Strauss syndrome. Allergy Asthma Respir Dis. 2015; 3:159–63.
Article17. Jeong HC, Kim KH, Cho JY, Song JE, Yoon HJ, Seon HJ, et al. Cardiac involvement of churg-strauss syndrome as a reversible cause of dilated cardiomyopathy. J Cardiovasc Ultrasound. 2015; 23:40–3.
Article18. Szczeklik W, Sokołowska BM, Zuk J, Mastalerz L, Szczeklik A, Musiał J. The course of asthma in Churg-Strauss syndrome. J Asthma. 2011; 48:183–7.
Article19. Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010; 125:1336–43.
Article20. Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017; 376:1921–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eosinophilic Granulomatosis with Polyangiitis: Experiences in Korean Patients
- Eosinophilic Annular Erythema in a Patient with Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss Syndrome)
- Eosinophilic granulomatosis with polyangiitis presenting with acutepolyneuropathy mimicking Guillain-Barré syndrome: A case report
- Eosinophilic Granulomatosis with Polyangiitis Diagnosed by Gallbladder Tissue
- Successful additional clarithromycin and tacrolimus treatment for hypereosinophilia associated with eosinophilic granulomatosis with polyangiitis