Brain Tumor Res Treat.
2019 Oct;7(2):85-91. 10.14791/btrt.2019.7.e33.
Efficacy of Slow Rate Ventriculolumbar Perfusion Chemotherapy for Leptomeningeal Carcinomatosis: Interim Result of a Phase II Study
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- 2Department of Cancer Control, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea. nsghs@ncc.re.kr
- 3Biometric Research Branch, National Cancer Center, Goyang, Korea.
- 4Neuro-Oncology Clinic, National Cancer Center, Goyang, Korea.
- KMID: 2461180
- DOI: http://doi.org/10.14791/btrt.2019.7.e33
Abstract
- BACKGROUND
To evaluate the efficacy of modified ventriculolumbar perfusion (VLP) chemotherapy with methotrexate on leptomeningeal carcinomatosis in terms of symptomatic response and side effects.
METHODS
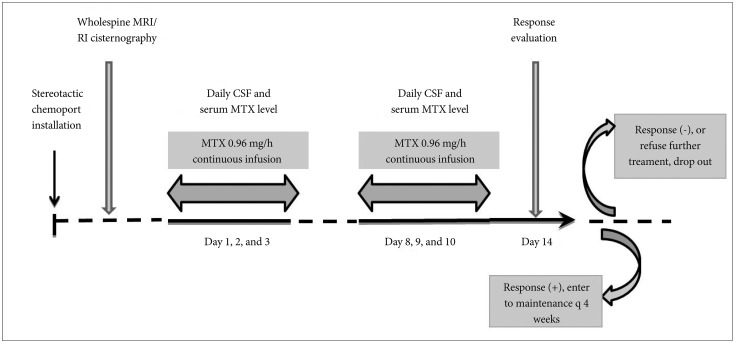
Previous infusion rate of 20 mL/h was reduced to 15 mL/h for the purpose of decreasing constitutional side effects of VLP such as nausea/vomiting, insomnia and confusion. The primary outcome was the response rate of increased intracranial pressure (ICP), and the secondary outcome was the occurrence of side effects compared to previous 20 mL/h trial. This interim analysis to validate the reduced infusion rate is not to affect the original effect of VLP chemotherapy.
RESULTS
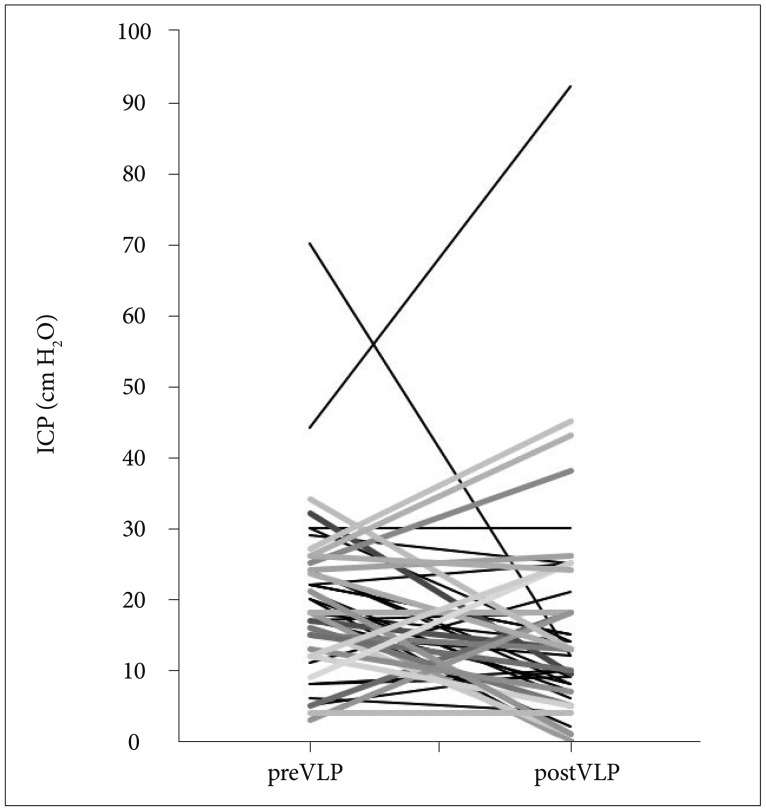
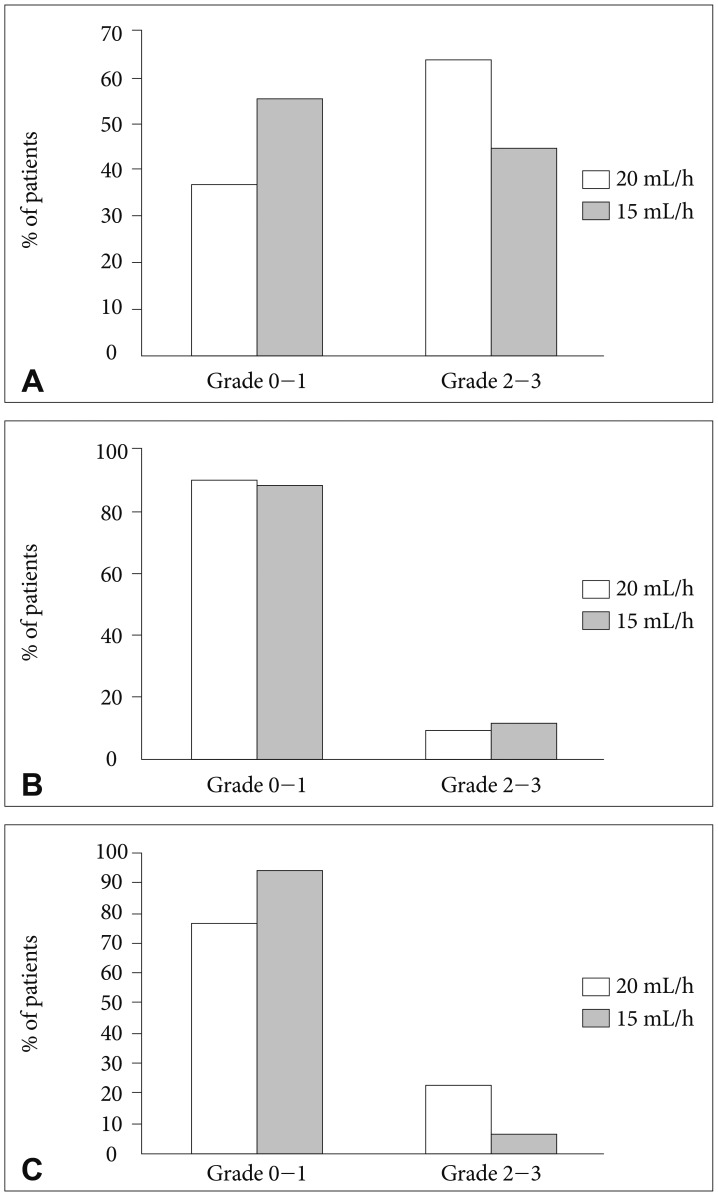
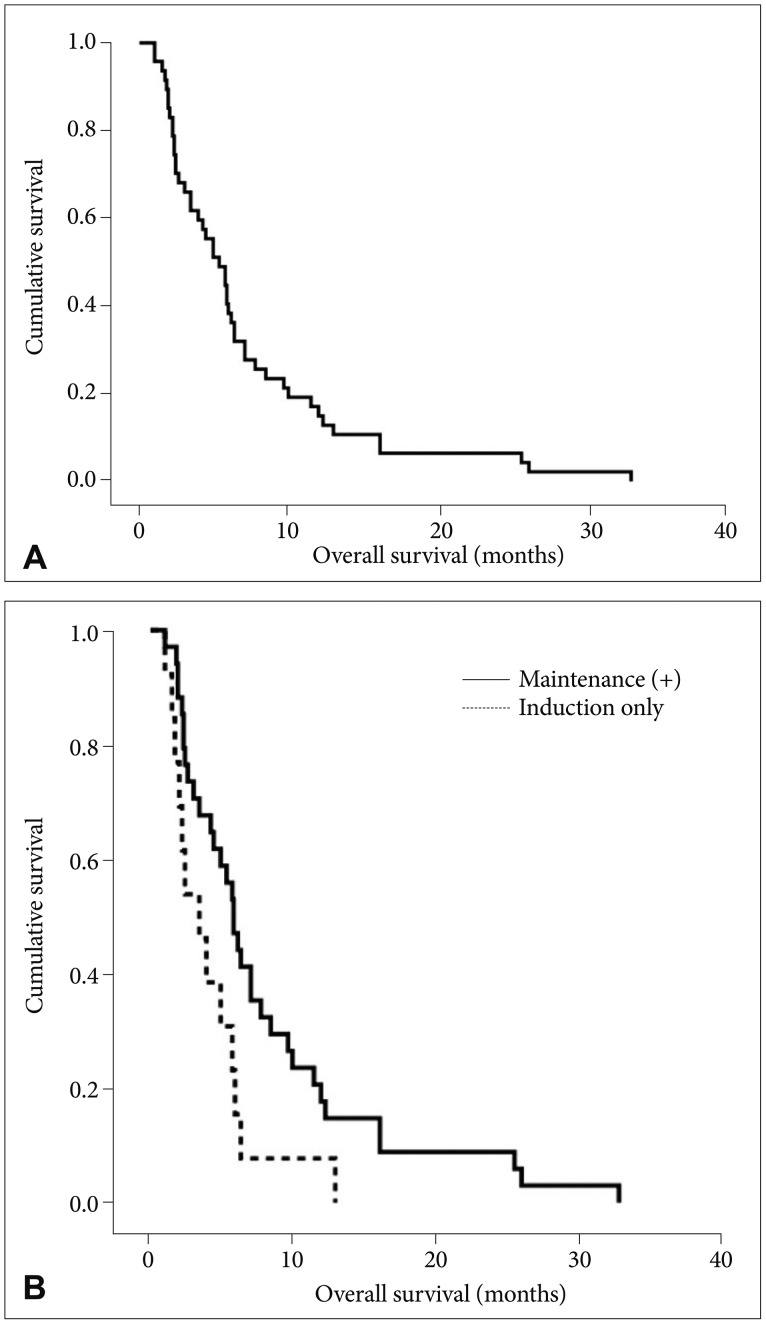
All forty-seven patients were enrolled including 22 patients with increased ICP. Thirteen patients out of these (59%) got normalized ICP after VLP chemotherapy. Moderate to severe (grade 2-3) confusion was observed in 3 patients (6%) and it was significantly reduced compared to those (23%) in the VLP 20 mL/h (p=0.017). Grade 2-3 nausea/vomiting was also reduced from 64% to 45% but failed to reach statistical significance (p=0.08). Median overall survival (OS) was 5.3 months (95% confidence interval, 3.55-7.05) and patients OS, who received maintenance VLP was significantly prolonged compared to patients who underwent induction VLP only (5.8 vs. 3.4 months, p=0.025).
CONCLUSION
VLP of reduced perfusion rate (15 mL/h) showed compatible control rate of increased ICP at this interim analysis. Decreased moderate to severe side effects and prolonged OS in patients received maintenance VLP encourage us to evaluate the effectiveness of this trial further.
Keyword
MeSH Terms
Figure
Reference
-
1. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982; 49:759–772. PMID: 6895713.
Article2. Chamberlain MC. Leptomeningeal metastases: a review of evaluation and treatment. J Neurooncol. 1998; 37:271–284. PMID: 9524085.3. Chamberlain MC, Kormanik P. Carcinoma meningitis secondary to non-small cell lung cancer: combined modality therapy. Arch Neurol. 1998; 55:506–512. PMID: 9561978.4. Sandberg DI, Bilsky MH, Souweidane MM, Bzdil J, Gutin PH. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery. 2000; 47:49–54. discussion 54-5. PMID: 10917346.
Article5. Grossman SA, Trump DL, Chen DC, Thompson G, Camargo EE. Cerebrospinal fluid flow abnormalities in patients with neoplastic meningitis. An evaluation using 111indium-DTPA ventriculography. Am J Med. 1982; 73:641–647. PMID: 6814249.6. Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998; 38:135–140. PMID: 9696363.7. Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. J Neurooncol. 2005; 75:85–99. PMID: 16215819.
Article8. Price RA, Jamieson PA. The central nervous system in childhood leukemia. II. Subacute leukoencephalopathy. Cancer. 1975; 35:306–318. PMID: 1089469.9. Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975; 293:161–166. PMID: 806016.
Article10. Blasberg RG, Patlak CS, Shapiro WR. Distribution of methotrexate in the cerebrospinal fluid and brain after intraventricular administration. Cancer Treat Rep. 1977; 61:633–641. PMID: 406996.11. Bleyer WA, Poplack DG, Simon RM. “Concentration x time” methotrexate via a subcutaneous reservoir: a less toxic regimen for intraventricular chemotherapy of central nervous system neoplasms. Blood. 1978; 51:835–842. PMID: 638249.
Article12. Balis FM, Blaney SM, McCully CL, Bacher JD, Murphy RF, Poplack DG. Methotrexate distribution within the subarachnoid space after intraventricular and intravenous administration. Cancer Chemother Pharmacol. 2000; 45:259–264. PMID: 10663645.
Article13. Rubin RC, Ommaya AK, Henderson ES, Bering EA, Rall DP. Cerebrospinal fluid perfusion for central nervous system neoplasms. Neurology. 1966; 16:680–692. PMID: 5949435.
Article14. Fleischhack G, Jaehde U, Bode U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin Pharmacokinet. 2005; 44:1–31.
Article15. Nakagawa H, Fujita T, Kubo S, et al. Ventriculolumbar perfusion chemotherapy with methotrexate and cytosine arabinoside for meningeal carcinomatosis: a pilot study in 13 patients. Surg Neurol. 1996; 45:256–264. PMID: 8638223.
Article16. Gwak HS, Lim HS, Shin SH, et al. Ventriculolumbar perfusion chemotherapy for the treatment of leptomeningeal carcinomatosis: a phase I study with pharmacokinetic data. Am J Clin Oncol. 2013; 36:491–499. PMID: 22781384.17. Gwak HS, Joo J, Shin SH, et al. Ventriculolumbar perfusion chemotherapy with methotrexate for treating leptomeningeal carcinomatosis: a Phase II Study. Oncologist. 2014; 19:1044–1045. PMID: 25209375.
Article18. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003; 13:176–181. PMID: 12903007.
Article19. Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995; 38:51–57. PMID: 7611725.
Article20. Gwak HS, Lee CH, Yang HS, et al. Chemoport with a non-collapsible chamber as a replacement for an Ommaya reservoir in the treatment of leptomeningeal carcinomatosis. Acta Neurochir (Wien). 2011; 153:1971–1978. PMID: 21796363.
Article21. Byun YH, Gwak HS, Kwon JW, et al. A novel implantable cerebrospinal fluid reservoir: a pilot study. J Korean Neurosurg Soc. 2018; 61:640–644. PMID: 30196661.22. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989; 10:1–10. PMID: 2702835.
Article23. Gwak HS, Joo J, Kim S, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol. 2013; 8:599–605. PMID: 23422833.
Article24. Chamberlain MC, Kormanik PA. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996; 46:1674–1677. PMID: 8649568.25. Bruna J, González L, Miró J, Velasco R, Gil M, Tortosa A. Neuro-Oncology Unit of the Institute of Biomedical Investigation of Bellvitge. Leptomeningeal carcinomatosis: prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009; 115:381–389. PMID: 19109820.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advancements of Treatment for Leptomeningeal Carcinomatosis
- Intrathecal Trastuzumab Treatment in Patients with Breast Cancer and Leptomeningeal Carcinomatosis
- A Case of Leptomeningeal Carcinomatosis with Bilateral Hearing Loss and Dizziness
- A Case of Leptomeningeal Carcinomatosis Presenting as a Neurological Complication of Stomach Cancer
- Response of Leptomeningeal Dissemination of Anaplastic Glioma to Temozolomide: Experience of Two Cases