Brain Tumor Res Treat.
2019 Oct;7(2):63-73. 10.14791/btrt.2019.7.e42.
The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade III Cerebral Gliomas in Adults: Version 2019.01
- Affiliations
-
- 1Division of Neurooncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 3Department of Neurosurgery, Bundang CHA Medical Center, CHA University, Seongnam, Korea.
- 4Department of Neurosurgery, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea.
- 5Department of Radiation Oncology, Ewha Women's University Mokdong Hospital, Ewha Women's University School of Medicine, Seoul, Korea.
- 6Department of Neurosurgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 7Department of Neurosurgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 8Department of Neurosurgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 9Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 10Department of Radiation Oncology, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 11Department of Neurosurgery, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- 12Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 13Division of Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 14Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 15Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 16Department of Neurosurgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 17Clinic of Pediatric Oncology, National Cancer Center, Goyang, Korea.
- 18Department of Neurosurgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 19Department of Neurosurgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 20Department of Neurosurgery, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea. dschung@catholic.ac.kr
- 21Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- 22Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 23Department of Radiation Oncology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 24Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 25Department of Neurosurgery, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Gwangju, Korea.
- 26Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 27Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 28Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 29Department of Neurosurgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 30Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dh8lim@skku.edu
- KMID: 2461178
- DOI: http://doi.org/10.14791/btrt.2019.7.e42
Abstract
- BACKGROUND
There was no practical guideline for the management of patients with central nervous system tumor in Korea in the past. Thus, the Korean Society for Neuro-Oncology (KSNO), a multidisciplinary academic society, developed the guideline for glioblastoma successfully and published it in Brain Tumor Research and Treatment, the official journal of KSNO, in April 2019. Recently, the KSNO guideline for World Health Organization (WHO) grade III cerebral glioma in adults has been established.
METHODS
The Working Group was composed of 35 multidisciplinary medical experts in Korea. References were identified by searches in PubMed, MEDLINE, EMBASE, and Cochrane CENTRAL databases using specific and sensitive keywords as well as combinations of keywords. Scope of the disease was confined to cerebral anaplastic astrocytoma and oligodendroglioma in adults.
RESULTS
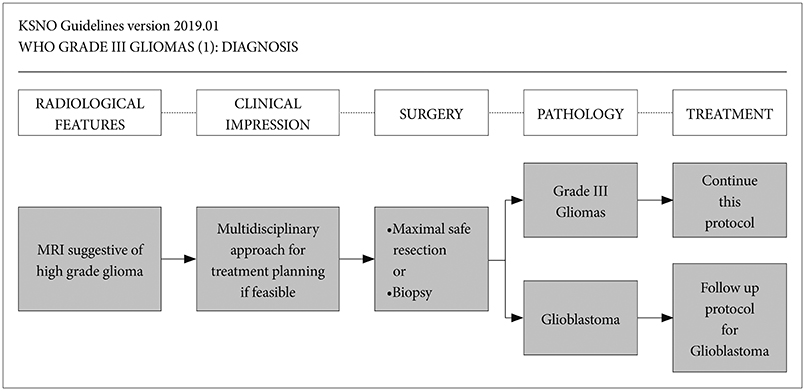
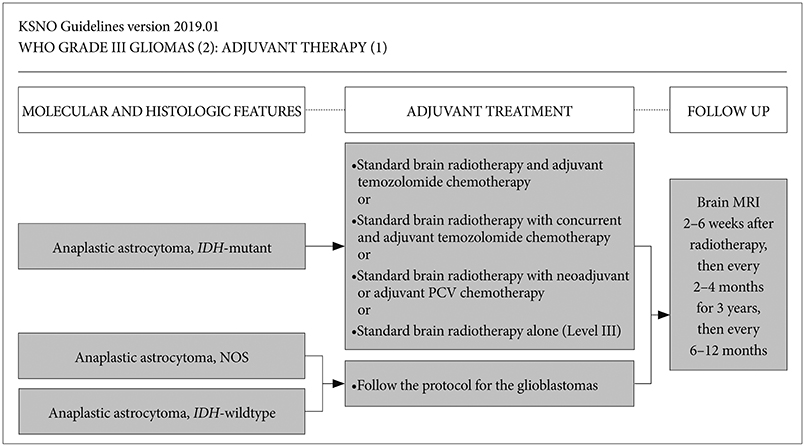
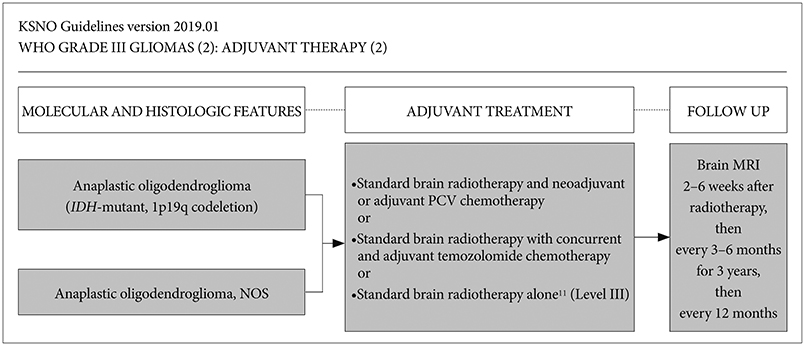
Whenever radiological feature suggests high grade glioma, maximal safe resection if feasible is globally recommended. After molecular and histological examinations, patients with anaplastic astrocytoma, isocitrate dehydrogenase (IDH)-mutant should be primary treated by standard brain radiotherapy and adjuvant temozolomide chemotherapy whereas those with anaplastic astrocytoma, NOS, and anaplastic astrocytoma, IDH-wildtype should be treated following the protocol for glioblastomas. In terms of anaplastic oligodendroglioma, IDH-mutant and 1p19q-codeletion, and anaplastic oligodendroglioma, NOS should be primary treated by standard brain radiotherapy and neoadjuvant or adjuvant PCV (procarbazine, lomustine, and vincristine) combination chemotherapy.
CONCLUSION
The KSNO's guideline recommends that WHO grade III cerebral glioma of adults should be treated by maximal safe resection if feasible, followed by radiotherapy and/or chemotherapy according to molecular and histological features of tumors.
MeSH Terms
Figure
Cited by 2 articles
-
The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO
Young Zoon Kim, Chae-Yong Kim, Do Hoon Lim
Brain Tumor Res Treat. 2022;10(2):83-93. doi: 10.14791/btrt.2022.0001.Korean Brain Tumor Society Consensus Review for the Practical Recommendations on Glioma Management in Korea
Chul-Kee Park, Jong Hee Chang
J Korean Neurosurg Soc. 2023;66(3):308-315. doi: 10.3340/jkns.2023.0046.
Reference
-
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018; 20(suppl_4):iv1–iv86.
Article2. Dho YS, Jung KW, Ha J, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23.
Article3. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
Article4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820.
Article5. Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology--Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014; 24:429–435.
Article6. Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015; 129:679–693.
Article7. van den Bent MJ, Klein M, Smits M, et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): a randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018; 19:1170–1179.
Article8. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017; 390:1645–1653.
Article9. Gwak HS, Yee GT, Park CK, et al. Temozolomide salvage chemotherapy for recurrent anaplastic oligodendroglioma and oligo-astrocytoma. J Korean Neurosurg Soc. 2013; 54:489–495.
Article10. Nabors LB, Portnow J, Ammirati M, et al. Central nervous system cancers, version 1.2015. J Natl Compr Canc Netw. 2015; 13:1191–1202.
Article11. Nabors LB, Portnow J, Ammirati M, et al. NCCN guidelines insights: central nervous system cancers, version 1.2017. J Natl Compr Canc Netw. 2017; 15:1331–1345.
Article12. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, central nervous system cancers. Version 1. 2019. Accessed August 1, 2019,. at https://www.nccn.org/professionals/physician_gls/default.aspx#cns.13. Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014; 15:e395–e403.
Article14. Kim YZ, Kim CY, Lim J, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for glioblastomas: version 2018.01. Brain Tumor Res Treat. 2019; 7:1–9.
Article15. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neurooncology working group. J Clin Oncol. 2010; 28:1963–1972.
Article16. Roh TH, Kang SG, Moon JH, et al. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: a retrospective study. J Neurosurg. 2019; 03. 01. DOI: 10.3171/2018.12.JNS182558. [Epub].
Article17. Van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010; 120:297–304.
Article18. Fuller CE, Schmidt RE, Roth KA, et al. Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J Neuropathol Exp Neurol. 2003; 62:1118–1128.
Article19. Weller M, Pfister SM, Wick W, Hegi ME, Reifenberger G, Stupp R. Molecular neuro-oncology in clinical practice: a new horizon. Lancet Oncol. 2013; 14:e370–e379.
Article20. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360:765–773.21. Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009; 118:469–474.
Article22. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015; 372:2499–2508.
Article23. Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009; 27:4150–4154.
Article24. Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010; 120:707–718.
Article25. Van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31:344–350.
Article26. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013; 31:337–343.
Article27. Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019; 393:678–688.
Article28. Lombardi G, De Salvo GL, Brandes AA, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019; 20:110–119.
Article29. Louis DN, Aldape K, Brat DJ, et al. Announcing cIMPACT-NOW: the consortium to inform molecular and practical approaches to CNS tumor taxonomy. Acta Neuropathol. 2017; 133:1–3.
Article30. Louis DN, Giannini C, Capper D, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018; 135:639–642.
Article31. Killela PJ, Pirozzi CJ, Reitman ZJ, et al. The genetic landscape of anaplastic astrocytoma. Oncotarget. 2014; 5:1452–1457.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO
- The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade II Cerebral Gliomas in Adults: Version 2019.01
- Perfusion MR Imaging in Gliomas: Comparison with Histologic Tumor Grade
- The Korean Society for Neuro-Oncology (KSNO) Guideline for the Management of Brain Tumor Patients During the Crisis Period: A Consensus Survey About Specific Clinical Scenarios (Version 2023.1)
- Yesterdays, Todays, and Tomorrows—Korean Society for Pediatric Neuro-Oncology*