Korean J Physiol Pharmacol.
2019 Nov;23(6):493-499. 10.4196/kjpp.2019.23.6.493.
Rhodanthpyrone A and B play an anti-inflammatory role by suppressing the nuclear factor-κB pathway in macrophages
- Affiliations
-
- 1College of Pharmacy, Woosuk University, Wanju 55338, Korea.
- 2Department of Pharmacology, School of Medicine, Wonkwang University, Iksan 54538, Korea.
- 3College of Pharmacy, Dankook University, Cheonan 31116, Korea.
- 4College of Pharmacy, Chonbuk National University, Jeonju 54896, Korea. ejbae7@jbnu.ac.kr
- KMID: 2461042
- DOI: http://doi.org/10.4196/kjpp.2019.23.6.493
Abstract
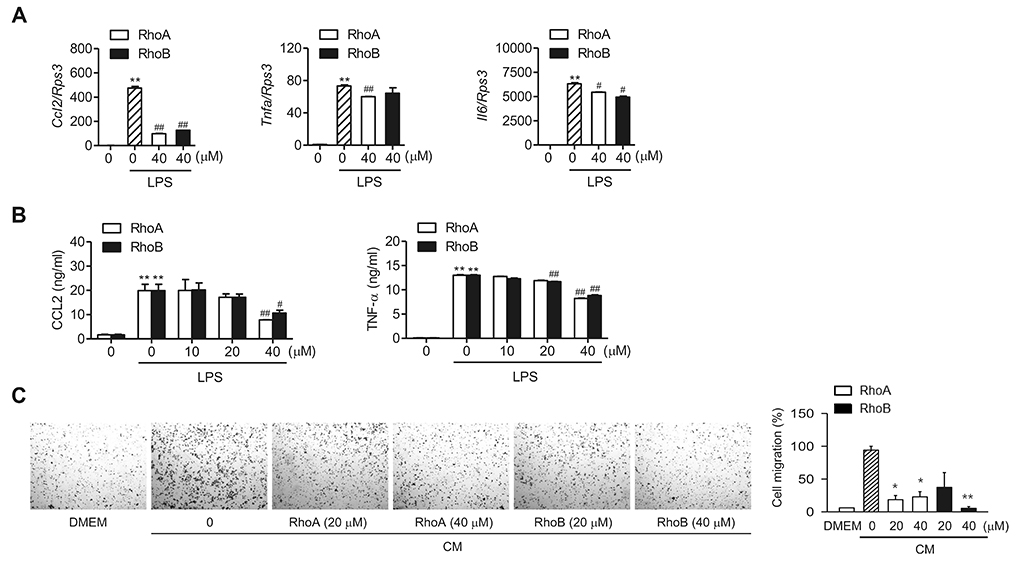
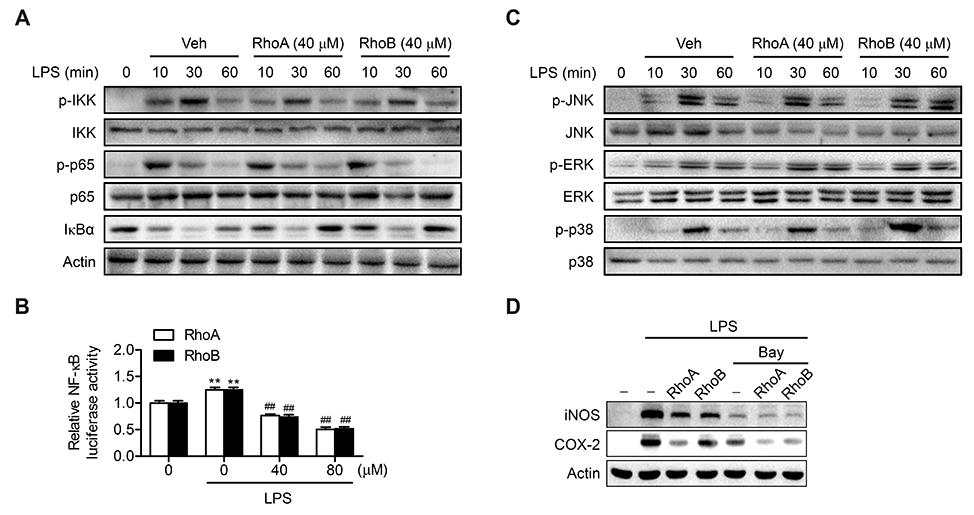
- Macrophage-associated inflammation is crucial for the pathogenesis of diverse diseases including metabolic disorders. Rhodanthpyrone (Rho) is an active component of Gentiana rhodantha, which has been used in traditional Chinese medicine to treat inflammation. Although synthesis procedures of RhoA and RhoB were reported, the biological effects of the specific compounds have never been explored. In this study, the anti-inflammatory activity and mechanisms of action of RhoA and RhoB were studied in lipopolysaccharide (LPS)-stimulated macrophages. Pretreatment with RhoA and RhoB decreased inducible nitric oxide synthase and cyclooxygenase-2 expressions in RAW 264.7 cells and in thioglycollate-elicited mouse peritoneal macrophages. In addition, it downregulated transcript levels of several inflammatory genes in LPS-stimulated RAW 264.7 cells, including inflammatory cytokines/chemokines (Tnfa, Il6, and Ccl2) and inflammatory mediators (Nos2 and Ptgs2). Macrophage chemotaxis was also inhibited by treatment with the compounds. Mechanistic studies revealed that RhoA and RhoB suppressed the nuclear factor (NF)-κB pathway, but not the canonical mitogen activated protein kinase pathway, in LPS-stimulated condition. Moreover, the inhibitory effect of RhoA and RhoB on inflammatory gene expressions was attenuated by treatment with an NF-κB inhibitor. Our findings suggest that RhoA and RhoB play an anti-inflammatory role at least in part by suppressing the NF-κB pathway during macrophage-mediated inflammation.
MeSH Terms
Figure
Reference
-
1. Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol. 2012; 22:R733–R740.
Article2. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013; 496:445–455.
Article3. Schultze JL, Schmieder A, Goerdt S. Macrophage activation in human diseases. Semin Immunol. 2015; 27:249–256.
Article4. de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015; 33:823–874.
Article5. Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009; 27:693–733.6. Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004; 382(Pt 2):393–409.7. Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994; 269:4705–4708.
Article8. Appleby SB, Ristimäki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994; 302(Pt 3):723–727.
Article9. Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001; 13:85–94.
Article10. Xu M, Zhang M, Wang D, Yang CR, Zhang YJ. Phenolic compounds from the whole plants of Gentiana rhodantha (Gentianaceae). Chem Biodivers. 2011; 8:1891–1900.
Article11. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010; 142:687–698.
Article12. Ka SO, Song MY, Bae EJ, Park BH. Myeloid SIRT1 regulates macrophage infiltration and insulin sensitivity in mice fed a high-fat diet. J Endocrinol. 2015; 224:109–118.
Article13. Young Taek H. A concise synthesis of rhodanthpyrone A and B, natural 4-(hydroxyphenyl)-substituted a-pyrones. Nat Prod Commun. 2017; 12:95–98.14. Wang Z, Ka SO, Han YT, Bae EJ. Dihydropyranoaurone compound damaurone D inhibits LPS-induced inflammation and liver injury by inhibiting NF-κB and MAPK signaling independent of AMPK. Arch Pharm Res. 2018; 41:314–323.
Article15. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–749.
Article16. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012; 18:363–374.
Article17. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018; 155:407–417.
Article18. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010; 72:219–246.
Article19. Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006; 103:2274–2279.
Article20. Kim HJ, Lee HS, Chong YH, Kang JL. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 2647 macrophages. Toxicology. 2006; 225:36–47.21. Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014; 26:253–266.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aloe-emodin inhibits Pam₃CSK₄-induced MAPK and NF-κB signaling through TLR2 in macrophages
- Beauvericin, a cyclic peptide, inhibits inflammatory responses in macrophages by inhibiting the NF-κB pathway
- Effect of Erythromycin on Pro-inflammatory Signalings by Particles
- Anti-Inflammatory Effect of Rosa rugosa Flower Extract in Lipopolysaccharide-Stimulated RAW264.7 Macrophages
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages